Preventive control plan templates for domestic food businesses
On this page
- 1. Template for ensuring the establishment is maintained and operated as required
- 2. Templates for conducting a hazard analysis
- 3. Templates to describe how you control your hazards
- 4. Template to describe the measures in place to meet consumer protection requirements
The document A guide for preparing a preventive control plan: Domestic food businesses explains what is generally included in a PCP and describes in a step-wise manner the process you can follow to develop your PCP. This guide is the first step to understanding what a PCP is and how to develop one.
The preventive control templates provided in this document are meant to demonstrate what domestic food businesses need to consider and what needs to be addressed in a PCP. The templates are generic and can be modified to suit your food business. Instructions for completing the templates, as well as completed examples, are provided within the document.
Keep in mind
The PCP requirements include measures related to food safety, consumer protection and animal welfare. This document provides guidance on how to prepare the food safety and consumer protection aspects of your preventive control plan.
Refer to the Guidelines for the humane care and handling of food animals during slaughter activities for information incorporating animal welfare measures into your preventive control plan.
There are four sets of templates for the preventive control plan:
- A template you can use to check that your establishment is maintained and operated to meet the applicable requirements in sections 50 to 81 of the Safe Food for Canadians Regulations (SFCR).
- Templates you can use to conduct a hazard analysis.
- Templates you can use to describe how you control your hazards, your critical control points (CCPs), and your verification procedures for all control measures.
- A template you can use to document the measures you have in place to meet the consumer protection requirements (for example, labelling, packaging, grading, standards of identity and net quantity) from the applicable sections referred to in 89(1)(a) and (b) of the SFCR.
Note: The preventive control requirements are found in Part 4 of the SFCR and requirements for the written preventive control plan are found in sections 86 through 89.
1. Template for ensuring the establishment is maintained and operated as required
You need to ensure that you operate and maintain your establishment according to the requirements in sections 50 to 81 of Part 4 of the SFCR so that the processing environment and personnel are not a source of contamination to the food.
The following checklist template is designed to help you confirm this.
It may be helpful to refer back to this checklist later on when you are determining the control measures you have in place for the identified hazards.
Maintenance and operation of establishment checklist
Here are suggestions for completing the template.
Is this adequately addressed?
- Check the appropriate box.
Justification
- Indicate how you do, or do not, meet the requirements.
Here is an example of a completed Maintenance and operation of establishment checklist: PDF (270 kb)
| SFCR section | Requirement | Is this adequately addressed? | Justification |
|---|---|---|---|
| 50 | Sanitation
|
Yes No |
|
| 63 | Lighting
|
Yes No |
|
2. Templates for conducting a hazard analysis
When preparing a preventive control plan, you are required to identify and analyze the biological, chemical and physical hazards that are associated with your food and process. The following four templates are designed to guide you through this.
- The Product description template outlines the details and characteristics of your foods, including the ingredients and packaging materials used.
- The Process flow diagram template outlines the sequence and interactions of each process step from receiving to final product shipping.
- The Traffic flow diagram template maps the movement of food and employees within the facility.
- The Hazard identification and evaluation template captures your hazard analysis.
The product description, process flow and the traffic flow templates provide a way for you to systematically identify potential hazards associated with inputs, processing steps and the movement of people and food.
Product description template
The Product description template begins the hazard analysis process by providing a complete description of the food prepared in your establishment. By completing this template you are describing the various characteristics of your final product, as well as how the food is packaged, stored and distributed. This information forms the basis for understanding the important food safety characteristics of the product and for identifying potential hazards.
Here are suggestions for completing the Product description template.
- Food name(s)
- Provide the name of the food. Include the brand name and common name.
- Important final product characteristics
- Identify the characteristics of the food that affect food safety.
- For example, pH, water activity (Aw), and salt content or concentration of preservatives.
- Identify the characteristics of the food that affect food safety.
- Ingredients and other inputs
- List every ingredient and any other input added or used during processing, including any relevant details (for example, source of fish (farmed or wild caught). Don't forget to include ingredients such as water and food additives or other inputs such as processing aids.
- Include the components of each ingredient, such as the ingredients of sauces and seasonings.
- Packaging
- List the packaging materials used.
- How the final product is being used
- State whether the food is ready-to-eat, to be cooked or if the food will be mixed with other foods for further processing.
- Shelf life
- Indicate the shelf life of the food under normal marketing conditions, including storage temperature (for example, kept frozen, refrigerated) and humidity if applicable.
- Where the food will be sold
- Describe where the food will be sold.
- Indicate if the food is being sold or marketed to a special group of consumers or institutions, such as infants or senior's homes and hospitals. This is important because certain populations (for example, the elderly, cancer patients, pregnant women) are especially vulnerable to specific food safety hazards.
- Labelling instructions
- List all labelling instructions for the food, such as any cooking or storage instructions.
- Distribution control
- List the controls required during transportation and storage, such as refrigeration.
Here is an example of a completed Product description template: PDF (17 kb).
| 1. Food name(s) | Frozen cooked peeled shrimp |
|---|---|
| 2. Important final product characteristics | Temperature < −18°C |
| 3. Ingredients and other Inputs | Raw farmed shrimp, glaze (salt, water) |
| 4. Packaging | Polyethylene bags 300g/800g |
| 5. How the final product is being used | Product is thawed and consumed without further cooking |
| 6. Shelf life | 6 months at −18°C |
| 7. Where the product would be sold | Domestic retail |
| 8. Labelling instructions | Keep frozen |
| 9. Distribution control | Keep at < −18°C |
Process flow diagram template
A process flow diagram lists the sequence of steps for producing and/or processing a food. A process flow diagram helps you identify process steps where hazards are reasonably likely to occur and where control measures would be most effective. It is important that the process flow include all steps, from receiving incoming materials to final product shipping, and highlights specific steps that are significant to food safety.
Here are suggestions for completing the Process flow diagram template.
Food name(s)
- Provide the name of the food covered by the process flow diagram.
Process flow diagram
- Use this box to draw out each of the process steps for the food.
- It is up to you to determine if a process flow diagram is needed for each food, or if foods made by similar processes can be combined into one diagram.
- It is recommended that you walk through the facility to verify that all of the steps are accounted for and accurate.
Note: Later, you will be able to indicate on the process flow diagram any CCP. They will be identified by using the second set of templates.
Here is an example of a completed Process flow diagram template : PDF (14 kb).
Food name: Breakfast cereal
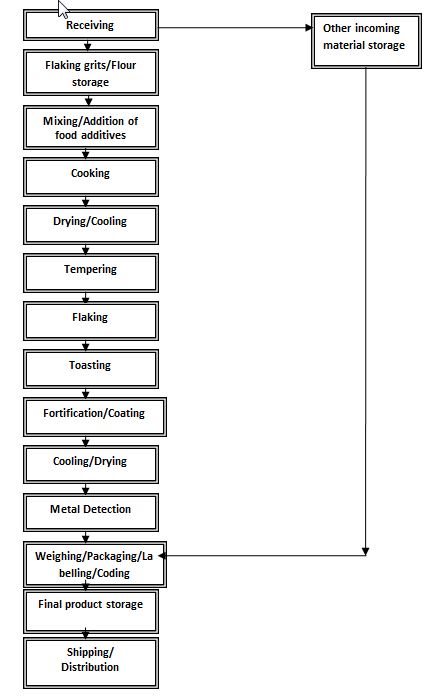
Description for the Process flow diagram
A process flow diagram shows the step-wise process for making a food. In this case the product being created is "breakfast cereal."
The "breakfast cereal" flow diagram starts with "Receiving," that breaks off into two paths.
The breakfast cereal follows several steps. The first step is "Flaking grits and flour storage." The second step is "Mixing and addition of food additives." The third step is "Cooking." The fourth step is "Drying and cooling." The fifth step is "Tempering." The sixth step is "Flaking." The seventh step is "Toasting." The eighth step is "Fortification and coating." The ninth step is "Cooling and drying." The tenth step is "Metal detection." The eleventh step is "Weighing, Packaging, labelling and coding." The twelfth and final step is "Final product storage" and finally "Shipping and distribution."
The second path from "Receiving" has four steps. The first step is "Other incoming material Storage." The second step is "Weighing, packaging, labelling and coding." The third step is "Final product storage." The fourth and final step is "Shipping and distribution."
Traffic flow diagram template
Traffic flow diagrams provide a basis for evaluating potential areas where food can be contaminated with hazards such as pathogens (illness causing germs), foreign materials, chemicals and allergens.
Traffic flow diagrams illustrate the flow of:
- raw foods and ingredients
- finished products
- workers, including to and from locker rooms, washrooms, and lunchrooms
- waste materials and inedible products
- chemicals
- allergens
Don't forget to include any areas where hand washing stations, hand sanitizers and boot cleaning stations are located.
Some of the potential cross contamination points you may identify during the hazard analysis process can be controlled by using sanitary zones or areas with restricted access for employees. The Traffic flow diagram template can be used to illustrate these areas.
Here are suggestions for completing the Traffic flow diagram template.
Food name(s)
- Provide the name of the food covered by the traffic flow diagram.
Traffic flow diagram
- Use this box to illustrate the floor plan of your facility (for example, plant schematic), indicating the various areas where people, equipment and food are located. For more complex floor plans you may wish to provide these details in a separate document. If so, refer to that document here.
- Use arrows to indicate the traffic flow patterns that people and food follow, and indicate the name of the traffic flow that is being illustrated.
- Mark potential cross contamination points on your diagram.
Here is an example of a completed Traffic flow diagram template: PDF (14 kb).
Food name: Ready-to-eat soup (with shrimp)
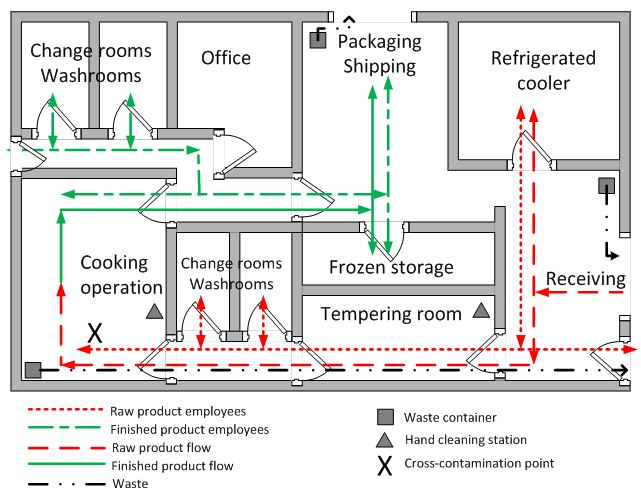
Description for flowchart: Traffic flow diagram
The Traffic flow diagram identifies various rooms in a facility, and the process in which a product would enter a facility in its raw form and then leave the facility in its processed form. There are two paths, raw products and employees, and finished products and employees.
The rooms identified in this diagram include the following: change rooms and washrooms, office, packaging and shipping, refrigerated cooler, receiving, frozen storage, tempering room, additional change rooms and washrooms, and cooking operation.
The raw product employee and raw product flow moves from the receiving area to the following areas: the refrigerated cooler, the tempering room, the cooking operation room. A waste container is located in the receiving area and the tempering room has a hand cleaning station. A waste container and a hand cleaning station are located in the cooking operation room.
Raw product employees have access to a change room and washroom located between the cooking operation and the tempering room.
The finished product employee flow moves from the cooking operation room to the frozen storage, to the packaging and shipping areas, and then the change room and washroom located beside the office.
The finished product flow moves from the cooking operation room to the frozen storage and then to the packaging and shipping area. There is a waste container located in the packaging and shipping area.
The waste in the cooking operation and receiving leaves via the exterior door in receiving. The waste in the packaging shipping room leaves through the exterior door in that room.
Hazard identification and evaluation template
This template can help you determine the biological, chemical and physical hazards that may be reasonably expected to pose a risk to your food and how you control those hazards. You do this by considering each input, processing step and cross contamination point.
Here are suggestions for completing the Hazard identification and evaluation template.
Food name(s)
- Provide the name of the food.
Input, process step or cross contamination point
- List all the inputs, processing steps and cross contamination points (one per row) from the Product description template, the Process flow diagram template, and the Traffic flow diagram template.
Hazard and cause
- List the hazards that may be reasonably expected to occur for each ingredient, input, processing step or cross contamination point, and the cause. Be specific.
- For example, pathogen survival (Listeria monocytogenes) due to insufficient time/temperature application.
Control measure
- Indicate how you control each identified hazard.
Is the hazard significant?
- Indicate (yes or no) if the potential hazard is significant, in other words if it is likely to occur and would severely affect the health of consumers if it was not controlled.
- Determining the significance of hazards helps you determine if there are any CCPs later on.
Justification
- Indicate your rationale for why the hazard is or is not significant.
Here is an example of a completed Hazard identification and evaluation template: PDF (19 kb).
| Input, process step or cross contamination point | Hazard and cause | Control measure | Is the hazard significant? | Justification |
|---|---|---|---|---|
| Ingredient: Raw farmed shrimp | (Chemical) Residues from drugs used at the shrimp farm to control disease, at levels above prescribed limits (Therapeutants) | Supplier's guarantee | No | Shrimps come from a trusted supplier, with a guarantee in place. |
| Process step: Storage | (Chemical, biological, physical) Contamination by various hazards during storage due to the presence of pests and/or contamination with cleaning chemicals. | Maintenance and operation of establishment (SFCR sections 50-81). | No | A "Maintenance and Operation of Establishment" program is implemented at our establishment to provide a sanitary environment for the production of food. |
| Process step: cooking | (Biological) Illness causing germs (pathogens) that aren't killed if the cooking time and temperature are insufficient | Adequate cooking time and temperature | Yes | If cooking time and temperature parameters are not met for every batch, there is a probability that illness causing germs (pathogens) could be present in the final product and consumers could get food poisoning. |
| Cross contamination point: Processing line | (Chemical) Contamination of food with allergens from other foods processed on the same line | Production line is cleaned and visually inspected prior to processing allergen-free food: See standard operating procedure (SOP) 1: processing line cleaning and sanitation and SOP2: pre-op inspection of processing line before dairy free soup | Yes | Very low levels of allergens can harm allergic consumers, and could be present in the food if an allergen control program is not strictly followed. |
3. Templates to describe how you control your hazards
Once you have identified where hazards could occur in your food and process and how you control them, you need to develop written procedures to describe your control measures in more detail. You also have to determine if any CCP exist for significant hazards, and set critical limits for each of them.
The following templates are designed to help describe your control measures, identify CCPs and their critical limits, and set up the corrective action procedures associated with your CCPs.
- Control measures template highlights the information you need to include to describe the control measures you have for the hazards associated with your food and process.
- Critical control point determination template examines the significant biological, chemical and physical hazards to determine if CCPs exist.
- Critical control point procedures template sets up the monitoring procedures and corrective action procedures for each CCP.
- Verification procedures for control measures template describes the procedures you have in place to ensure your control measures are implemented and work as intended.
Control measures template
The Control measures template highlights the information you need to include to describe the control measures you have for the hazards associated with your food and process.
Here are some suggestions for completing the Control measures template.
Food name(s)
- Provide the name of the food.
Control measure
- Indicate the control measure(s) identified in the Hazard identification and evaluation template.
Description
- Describe each control measure by including details such as:
- The task(s) required to carry out the control measure. For more complex control measures you may wish to provide these details in a separate document (for example, a standard operating procedure). If so, refer to that document in this box.
- How frequently the tasks are carried out. Be specific (for example, hourly, weekly, monthly).
- The job title of the person responsible for the control measure.
Documents
- It is a good practice to list any forms you use for the day-to-day collection of information used to record delivery of tasks and controls. It allows you to keep track of all the documents related to your written preventive control plan.
Here is an example of a completed Control measures template: PDF (17 kb).
| Control measure | Description | Documents |
|---|---|---|
| Supplier's guarantee | You require a signed contract with shrimp farm owner that guarantees shrimps do not contain residues from drugs used to control disease, at levels above prescribed limits, and outlines the other conditions (for example, lab testing) to be met by the shrimp farm. Renegotiate yearly: Include this requirement in regular contract renewals. |
Contract on file. Lab reports from supplier (every 10 lots). |
Critical control point determination template
The Critical control point determination template lists the process steps where you have identified a significant hazard(s) and provides a series of questions that guide you through the process of identifying CCPs.
Here are suggestions for completing the Critical control point determination template.
Food name(s)
- Provide the name of the food.
Process step
- List each process step where a significant hazard was determined on the Hazard identification and evaluation template.
Significant hazards
- Transfer the significant hazard(s) associated with the process step, including its cause, from the Hazard identification and evaluation template.
- Where you have identified significant hazards for inputs (ingredients and packaging) or contamination points on the Hazard identification and evaluation template, describe these hazards on the Critical control point determination template at the processing step where they are introduced.
- For example, if you have identified a pathogen as a hazard in an ingredient, include this hazard at the process step "receiving" if this is the point where the ingredient is introduced into your process.
Then, answer the following questions for each significant hazard:
Q1. Do control measures for this hazard exist at this step?
- Indicate yes or no.
- If yes, proceed to Q2.
- If no, this is not a CCP. Indicate the step where and how the hazard will be controlled. Proceed to the next process step.
Q2. Is this step specifically designed to prevent or eliminate the hazard or reduce it to an acceptable level?
- If yes, this is a CCP. Proceed to the last column.
- If no, proceed to Q3.
Q3. Will a subsequent step eliminate the hazard or reduce it to an acceptable level?
- If yes, this step is not a CCP. Identify the subsequent step where the hazard will be controlled.
- If no, this step is a CCP and must be designed to control the hazard. Proceed to the last column.
CCP number
- Number the CCP and identify the type of hazard (B for Biological, C for Chemical or P for Physical).
- For example, CCP1B.
- Indicate the CCP number on the Process flow diagram beside the corresponding process step.
Here is an example of a completed Critical control point determination template: PDF (77 kb).
| Process step | Significant hazards | Q1. Do control measures for this hazard exist at this step? | Q2. Is this step specifically designed to prevent or eliminate the hazard or reduce it to an acceptable level? | Q3. Would a subsequent step eliminate the hazard or reduce it to an acceptable level? | CCP number |
|---|---|---|---|---|---|
| Describe hazard and cause | If yes, proceed to Q2. If no, this is not a CCP. Indicate where and how the hazard will be controlled. Proceed to the next process step. |
If yes, this is a CCP. Proceed to the last column. If no, proceed to Q3. |
If yes, this step is not a CCP. Identify the subsequent step where the hazard would be controlled. If no, this step is a CCP and must be designed to control the hazard. Go to last column. |
Number the CCP and identify the type of hazard (B, C or P) | |
| Mixing, cooking, packaging | (Chemical) Contamination of food with fish allergens from other foods processed on the same line | No: controlled by Standard Operating Procedure (SOP) 1: Processing line cleaning and sanitation, and SOP 2: Pre-op inspection of processing line before dairy free soup | |||
| Cooking | (Biological) Pathogen survival if cooking time and temperature are insufficient | Yes | Yes | CCP#1B |
Critical control point procedures template
For every CCP, you need to:
- establish the critical limits,
- set monitoring procedures for CCPs to ensure that the critical limits are continuously met, and
- set corrective action procedures for when critical limits are not met.
Here are suggestions for completing the Critical control point procedures template.
Food name(s)
- Provide the name of the food.
CCP
- Transfer the CCP number that you have identified on the Critical control point determination template.
Significant hazard
- Transfer the significant hazard(s) that is being controlled at that CCP from the Critical control point determination template.
Control measure
- Indicate the control measure(s) that is preventing, eliminating the hazard, or reducing it to an acceptable level.
- Note that you should have already identified this control measures in the Control measures template. Since you have identified that this control measure is being applied at a CCP in the Critical Control point determination template, more details are required as outlined in this template.
Critical limits
- Indicate the critical limits for the control measure at the CCP.
Monitoring procedure
- Describe your procedure for monitoring the critical limits for each CCP.
- Include the following details in your procedure:
- What
- Indicate what you are monitoring.
- How
- Describe the monitoring activity.
- For more complex monitoring activities, you may wish to provide these details in a separate document. If so, refer to that document here.
- When
- Set a frequency for carrying out the monitoring activity.
- Monitoring can be continuous or intermittent. If monitoring is intermittent, the frequency must be sufficient to guarantee the CCP is in control.
- Who
- Name the job title of the person responsible for the monitoring activity.
- Records
- List any forms you use to record your monitoring activity at the CCP.
- What
Corrective action procedure
- Describe the actions to be taken when monitoring indicates a deviation from the critical limits.
- Include details on how you control the affected product and how you correct the problem that led to the deviation.
- Evaluate if other products could have been impacted and also need to be controlled.
- Keep records of all corrective actions.
Here is an example of a completed Critical control point procedures template: PDF (18 kb).
| Critical control point (CCP) | Significant hazard | Control measure | Critical limits | Monitoring procedure | Corrective action procedure |
|---|---|---|---|---|---|
| CCP#1B | (Biological) Pathogen survival if cooking time and temperature are insufficient | Heat process that achieves Listeria cook (5 log reduction of Listeria) | Batch of product must be 200L maximum Cooking for 5 minutes at 100C minimum (as per validation study) |
What:
When: every batch Who: Cooker operator Records: Cook log |
Records: CCP Corrective Action Log |
Verification procedures for control measures template
You need to describe the verification procedure for each control measure you listed in the Control measures template. A verification procedure is an explanation of how you ensure that the control measure is consistently implemented and effective at controlling the hazard.
Here are suggestions for completing the Verification of control measures template.
Food name(s)
- Provide the name of the food.
Name of the control measure to be verified
- Provide the name of each control measure from the Control measures template in this box.
- You may wish to group a series of related control measures together as part of a more comprehensive verification procedure. Ensure the template is completed in a way that clearly demonstrates everything that is being verified.
Verification procedure
- Describe the verification procedure for each control measure (or group of control measures).
- Include the following details in your verification procedure:
- How the control measure is verified, for example,
- visual inspections and employee interviews
- testing food contact surfaces, ingredients, final products
- dismantling equipment to verify the effectiveness of the cleaning process
- record reviews
- what to do when the control measure is not consistently implemented or is not effective.
- How the control measure is verified, for example,
- For more complex verification procedures, you may wish to provide these details in a separate document. If so, refer to that document here.
When
- Determine a verification frequency that allows you to discover potential issues before they affect food safety. For example, the frequency would be higher for control measures that are applied at CCPs.
Who
- Name the job title of the person responsible for the verification activity
- Ideally, this is a different person than the person that carried out the control measure.
Records
- List any forms you use to record your verification activities.
Here is an example of a completed Verification procedures for control measures template: PDF (16 kb).
| Name of control measure(s) to be verified | Verification procedure | When | Who | Records |
|---|---|---|---|---|
Supplier's guarantee |
Task 1:
Task 2: |
Task 1: Task 2: |
Task 1: Quality Assurance Manager Task 2: Owner and Quality Assurance manager |
Verification activities are recorded on Supplier Audit Checklist |
CCP#1B–Heat process that achieves Listeria cook (5 log reduction of Listeria) |
Task 1:
Task 2:
Task 3:
In case of non-conformity: Follow up actions are taken immediately (for example, hold affected product, operator re-training, re-calibrate thermometer, review of time temperature recorder calibration procedure). |
Task 1: Task 2: Task 3: |
Quality Assurance Manager | Verification activities are recorded on Verification Form |
4. Template to describe the measures in place to meet consumer protection requirements
In addition to the food safety requirements of Part 4 of the SFCR, you need to describe the measures that you have in place to demonstrate that you meet applicable requirements for labelling, packaging, standards of identity, grades, and net quantity for your foods – these are referred to as consumer protection requirements in this document.
You can find the specified requirements in section 89(1)(a) and (b) of the SFCR.
The measures for consumer protection requirements template is designed to help you capture these descriptions.
Measures for consumer protection requirements template
Here are suggestions for completing the template.
Food name(s)
- Provide the name of the food.
SFCR section
- Provide the applicable section number that you are describing a measure for.
Requirement
- Specify the requirement to be met.
Measure
- Describe the measures you use to meet each consumer protection requirement that is applicable to your food.
- For more complex measures you may wish to provide these details in a separate document (for example, a standard operating procedure). If so, refer to that document here.
Here is an example of a completed Measures for consumer protection requirements template: PDF (16 kb).
| SFCR section | Requirement | Measures |
|---|---|---|
| section 201 | Food must meet the applicable standard of identity |
|
- Date modified: