Canada Organic Regime operating manual
On this page
- Preface
- General information
-
Part A Assessment and designation of conformity verification bodies (CVB)
- A.1 Objective
- A.2 Requirements for entering into an agreement
- A.3 Initial assessment and designation of the CVB also CVB reassessments
- A.4 Monitoring and surveillance of CVB
- A.5 Renewal of the agreement between CFIA and CVB
- A.6 Breach of the agreement, suspension and early termination
- A.7 Complaints against CVBs
- A.8 CVB documents required for initial assessment
- A.9 Canada Organic Regime assessment cycle
- A.10 Annual information from the designated CVBs
-
Part B Accreditation of certification bodies (CB)
- B.1 Objective
- B.2 Initial CB accreditation
- B.3 Monitoring and surveillance of a CB
- B.4 Reassessments of a CB
- B.5 Appeals of CFIA accreditation decision by a CB
- B.6 Appeals of CVB recommendation decision of a CB
- B.7 Reduction of scope, suspension and cancellation of a CB
- B.8 Complaints against CB
- B.9 Records maintained by the CVB
- B.10 CVB agreement with the CB
- B.11 CB documents required for accreditation application (except the cases when a CB changes their CVB)
- B.12 Requirements when a CB changes CVB under the COR
- B.13 Requirements for voluntary withdrawal of a CFIA accredited CB under the COR
- B.14 Requirements when a CB goes out of business
- B.15 Requirements when a CVB can conduct remote audits at CBs
-
Part C Certification of organic product and CB requirements
- C.1 Objective
- C.2 Procedures for certification under COR
- C.3 Timing of sale or distribution of certified product
- C.4 Complaint and appeal
- C.5 Issues regarding implementation of the standard
- C.6 Use of licenses, certificates and marks of conformity
- C.7 Obligations of the CB relative to certifications
- C.8 Records control by the CB and operator
- C.9 CB records
- C.10 Requirements when an operator changes a CB under COR
- C.11 Requirements when a CB issues attestation of compliance
- C.12 Requirements for grower group certification under COR
- Appendix A - Certificate template - Informative
- Appendix B – Attestation of compliance template - Informative
- Appendix C - The family of certification documents
- Appendix D - CB management of nonconformities and enforcement actions under the Canada Organic Regime
- Appendix E - CVB Management of nonconformities and enforcement actions under the Canada Organic Regime (Initial Application/Reassessment for Accreditation (First part))
- Appendix F - CVB Management of nonconformities and enforcement actions under the Canada Organic Regime (Surveillance and Monitoring / Reassessment for Accreditation (Second part))
- Appendix G - Letter of good standing for an operator changing CB under the Canada Organic Regime (Template) - Informative
Preface
This Canada Organic Regime (COR) operating manual contains policies and procedures for activities applicable to the COR. The manual provides an overview of the procedure to be followed when the Canadian Food Inspection Agency (CFIA) and conformity verification bodies (CVB) enter into an agreement, as well as the procedure to accredit certification bodies (CB) and to certify organic products. The goal in producing this manual is to provide a structure so that services are delivered in a consistent and efficient manner.
Part 13 of the Safe Food for Canadians Regulations (SFCR) were made pursuant to section 51 of the Safe Food for Canadians Act. The purpose of the SFCR is to establish a system by which the CFIA as the competent authority in Canada for organic products marketed in interprovincial, export and import trade shall regulate the use of the "Canada Organic Logo" and organic claims.
The SFCR would facilitate international market access, provide more specific protection to consumers against deceptive and misleading labelling practices through a uniform approach to organic product certification and labelling, and support further development of the domestic market. The need for a federal regulatory regime has been identified and supported by the Canadian organic industry.
The COR CFIA envisions reviewing and amending the COR operating manual every 5 years. The CFIA might decide to review the COR Operating Manual earlier in cases of outstanding findings from peer reviews, changes to part 13 of the current SFCR or International requirements.
Should there be any discrepancy between the COR operating manual and the SFCR, the SFCR shall take precedence.
General information
Overview of the Canada Organic Regime (COR)
The COR is a non-traditional regime for the CFIA. The SFCR provide a federal program for the regulation of Canadian organic products. The COR is designed to build on the existing system of domestic accreditation and certification. The CFIA is the competent authority that oversees the COR governing the use of the Canada Organic Logo. The CFIA enters into agreements with Conformity verification bodies (CVBs) provided these bodies meet the criteria established by the SFCR and CFIA. For the purpose of the SFCR, the CVBs are designated by the CFIA to assess, recommend for accreditation and subsequently monitor certification bodies (CB) meeting the applicable accreditation criteria as set out in the SFCR.
The accredited CBs are responsible for the organic certification of food commodities and organic product packaging and labelling certification. CBs employ inspectors to assess the practices of organic operators to verify that they comply with the regulations. These inspectors are referred herein as verification officers (VO). The VO provides the results of their assessment to their CB for evaluation. The CB, in turn, certifies as organic only those products that comply with requirements of the regulations.
In order to facilitate the import/export activities and to verify that importing country requirements are equivalent or in compliance with the COR, an equivalency determination between Canada and another country shall be performed. Such determination may result in reducing the importing country's rate of verification and avoid additional certification in the country of origin.
The CFIA is responsible for compliance verification and enforcement of the regulations which activities include label inspections in the marketplace and audits of CVBs.
Building on the existing organic certification system, the SFCR set out the functions of the COR's 2 oversight bodies: CVBs and CBs.
In this document, the word "days" is only used when directly referencing a part of the SFCR that explicitly mentions a specified number of days (COR OM B.5.1-SFCR section 362, COR OM B.7.9-SFCR subsection 364(5) and COR OM B.7.10-SFCR paragraph 365(1)(a)). SFCR's use of "days" is interpreted as and understood to be "calendar days". All other parts of this document clearly specify "working days".
References
The documents listed below are those referenced by this document. At the time of publication, the editions indicated below were valid. As all documents are subject to revision, parties using this document are encouraged to apply the most recent editions of these documents published.
Additional accreditation criteria for bodies that evaluate quality management systems in companies whose operations are on multiple sites in order to certify their products – CAEQ, 2007
CAN/CGSB-32.310, Organic production systems general principles and management standards (to the extent these standards are incorporated by reference into the regulations and developed by the organic industry and the Canadian General Standards Board)
CAN/CGSB-32.311, Organic production systems permitted substances list (as incorporated by reference into the regulations and developed by the organic industry and the Canadian General Standards Board)
CAN/CGSB-32.312, Organic production systems: Aquaculture - General principles, management standards and permitted substances list (as incorporated by reference into the regulations and developed by the organic industry and the Canadian General Standards Board)
Certifying operations with multiple production units, sites and facilities under the National Organic Program, formal recommendation by the National Organic Standards Board (NOSB) to the National Organic Program, 2008
European Commission, Directorate H. sustainability and quality of agriculture and rural development, H.3 organic farming, Guidelines on imports of organic products into the European Union, Rev 1 dated 15.12.2008
IFOAM requirements for grower groups
ISO/IEC 17011: 2017 - Conformity assessment - Requirements for accreditation bodies accrediting conformity assessment bodies
ISO/IEC 17065: 2012 - Conformity assessment – Requirements for bodies certifying products, processes and services
SOR/2018-108, part 13 of the Safe Food for Canadians Regulations (regulations made under the authority of the Safe Food for Canadians Act
Definitions
- Accreditation cycle
- The period including the initial assessment or reassessment and the subsequent surveillance years
- Act
- The Safe Food for Canadians Act
- Agency
- The CFIA established by section 3 of the Canadian Food Inspection Agency Act responsible for the administration of the COR.
- Audit
- A systemic and independent examination to determine whether activities and related results comply with planned arrangements and whether these arrangements are implemented effectively and are suitable to achieve objectives.
- Canada Organic Logo
- "Product legend" as per SFCR Schedule 9, Clauses 350(1)(c)(i)(B) and (D), paragraph 354(d) and sections 358 and 359.
- Canada Organic Regime (COR)
- The Government of Canada regulated system for organic products.
- Certification
- The procedure whereby a CFIA accredited certification body provides written assurance that food commodities are organic as defined in and for the purposes of the SFCR. Certification of products may be based on a range of inspection activities including verification of management practices, auditing of quality assurance systems, and in/out production balances.
- Certification Body (CB)
- A body that is accredited as a CB in accordance with division 8 of part 13 of the SFCR, and CFIA shall accredit the applicant as a CB on the recommendation of the CVB.
- Compliance
- Adherence with requirements of laws and government regulations, for example, part 13 of the SFCR.
- Conformity Verification Body (CVB)
- An entity that shall meet the requirements set out in ISO/IEC 17011 to be able to enter into an agreement with the CFIA under subsection 14(1) of the Canadian Food Inspection Agency Act to assess, recommend the accreditation of and monitor the CB.
- Genetically engineered /modified organisms (GMO)
- Products produced through techniques in which the genetic material has been altered in a way that does not occur naturally by mating and/or natural recombination.
- Geographical proximity (with reference to grower group certification)
- Access to the same collection or post-harvest handling facilities, and/or common soils, water source, slope, topography or other physical features.
- Group certification
- Certification of an organized group of producers with a central office, similar farming and production system working according to a common internal quality management system, which is established and subject to continued surveillance by the central office. Group certification applies to the group as a whole. Certificate is issued to the central office of the group and shall not be used by a single group member.
- Internal Control System (ICS)
- A documented internal quality system within a grower group that allows an external CB to delegate the annual inspection of any group members to an identified body or unit within the grower group.
- Investigation
- Involves the gathering of evidence and information, from a variety of sources, relevant to a suspected violation or offence and is intended to refute the defence of due diligence and/or establish intent.
- Multi-ingredient product
- A type of food commodity composed of 2 or more food commodities.
- Nonconformity
- Occurrence of a condition that does not conform to the specifications of the prescribed standards (CAN/CGSB-32.310, CAN/CGSB-32.312, CAN/CGSB-32.311, ISO 17065, ISO 17011 and Part 13 of the SFCR).
- Organic product
- An food commodity that has been certified as organic in accordance with part 13 of the SFCR or that has been recognized as such under section 2 of the Safe Food for Canadians Act.
- Opportunity for Improvement (OFI)
- OFI is an opportunity to improve the organization's operating efficiency however if not addressed it might lead to a future nonconformity.
- Part 13 of the Safe Food for Canadians Regulations (SFCR)
- These are the regulations referred to throughout the COR Operating Manual.
- Small farm (with reference to grower group certification)
- Both: a. farm where the cost of external certification is 2% or more of their annual gross revenue, and b. less than 50 acres.
- Verification audit
- The purpose of a verification audit conducted by the CVB is to check the accuracy and thoroughness of the most recent inspection although it also offers an opportunity for an operator to comment on his inspection or the overall performance of the certification body should they wish to do so. In a verification audit, the CVB auditor repeats part or the whole of the physical inspection in company with the operator and compares any findings with that of the inspector as recorded in the inspection report. Short, partial checks of records are usually included as well.
- Verification officer (VO)
- Person assigned by the certification body to conduct inspections and having the requisite qualifications and experience to conduct inspections for the purposes of the regulations.
- Witness audit (by CFIA)
- The COR audit team witnesses the activities of the CVB auditor during the initial assessment and the monitoring of a CB.
- Witness audit (by the CVB)
- The purpose of a witness audit conducted by the CVB is to assess the quality of an inspector's performance through observing an inspection in process. An evaluation is made of the degree to which the inspector follows the policies and procedures of the certification body with regard to the application of scheme requirements by the operator. It is also an opportunity to observe the thoroughness with which inspectors investigate issues and the degree to which they are familiar with the reference standard. Auditors should remain silent during a witness audit although inspectors may be questioned further in private following the conclusion of the inspection. Inspectors should at any rate receive a short exit interview during which they may clarify any unclear issues and are informed of any points that the auditor intends to raise in the report.
Revision history
| Version | Date | Reason for the revision | Scope of the revision |
|---|---|---|---|
| - | Feb 2007 | Draft of the Canada Organic Office Quality Management System (COO QMS) manual sent for peer review | The entire document |
| - | Jun 20, 2007 | Feedback from the peer review incorporated in the COO QMS Manual | The entire document |
| - | Sep 11, 2007 | Feedback from the consultation with the industry | The entire document |
| - | Oct 2008 | CFIA comments | Part A |
| - | Jan 30, 2009 (not released) | Amendments to the 2006 Organic Products Regulations | The entire document |
| V11 | Nov 2009 (not released) | Consultation with the CVBs |
|
| V12 | Dec 2009 (not released) | Additional comments from the CVBs |
|
| V13 | Jun 11, 2010 | Edited for style and numbering of document. Include comments from CVBs and CFIA. New part on grower group requirements. New part on the Standards Interpretation Committee. |
|
| V14 | June 26, 2012 | Many editorial changes, addition of some new clauses and requirements | The entire document. |
| V15 | March 12, 2018 | Many editorial changes. |
|
| V16 | January 15, 2019 | Removal of part D and updates to part C due to SFCR coming into force. |
|
| V17 | April 04, 2019 |
|
|
| V18 | July 2020 | Added 3 new appendices (E, F and G) to provide clarification on the interpretation of part 13 of the SFCR. Minor editorial revisions throughout parts B and C. | Part B and C, appendices |
| V18 (revised) | January 2021 | Text corrected in 1st policy step | Appendix F |
| V18 (revised) | February 2021 | "The applicable type of certification" updated to match Canada Organic Standard wording | C.2.4.4 |
| V18 (revised) | June 2021 | Various edits to text | A.3, A.3.4, A.4.7-A.12, B.3.13, C.2.4.4 (3rd bullet), and deleted A.13-A.14 |
| V18 (revised) | July 2021 |
|
A.6.17, B.7.9, B.7.10, B.7.11, C.2.3.1 |
| V18 (revised) | June 2022 | All references to "days" are now "working days" | Appendix D |
| V19 | June 2023 | Significant changes to sections on grower group, certification C.2.4.4, and certificate template. All references to "days" are now "working days" except for B.5.1, B.7.9 and B.7.10. Minor editorial revisions throughout parts A, B and C and appendices. | Parts A, B, C and appendices |
| V19 (revised) | September 2023 | Wording update to C.2.4.4 and Appendix A | C.2.4.4 and Appendix A |
| V19 (revised) | January 2024 | Update to text B.3.18.2.3.3, section C.2.2 Application evaluation, C.2.4.4, C.2.5 Procedure for continuation of certification, C.12.4.2 | B.3.18.2.3.3, C.2.2 Application evaluation, C.2.4.4, C.2.5 Procedure for continuation of certification, C.12.4.2 |
Part A Assessment and designation of conformity verification bodies (CVB)
These requirements apply to the conformity verification body's (CVB) accreditation services provided for the purposes of the SFCR. Participation in the COR accreditation program is not intended to prevent CVBs from carrying out other business activities, especially those involving the accreditation of CBs not covered by the SFCR.
Operations resulting from these other activities should neither constitute an infringement nor result in conflicts of interest with the accreditation activities performed by the CFIA.
Further consideration shall be given to address COR requirements that shall be additional to those required by ISO/IEC 17011.
A.1 Objective
To outline the process under which the CFIA shall enter into an agreement with a CVB and maintain it.
A.2 Requirements for entering into an agreement
Only entities that meet the requirements set out in ISO/IEC 17011 may enter into agreement with the CFIA to assess, recommend for accreditation and monitor certification bodies.
A.3 Initial assessment and designation of the CVB also CVB reassessments
A.3.1 Application and documents screening
- A.3.1.1 Any applicant seeking information from the CFIA regarding the conditions under which CFIA shall enter into an agreement with a CVB may consult the CFIA website and access information from the CFIA organic products homepage.
- A.3.1.2 Upon request, the CFIA sends to the applicant an information package which contains a list of documents to be provided to initiate an assessment.
- A.3.1.3 The applicants may be either private or government entities.
- A.3.1.4 The applicant shall submit to the CFIA the documents listed in section A.8.
- A.3.1.5 The CFIA (COR team) reviews for adequacy the information supplied by the applicant and sends acknowledgement of receipt within 5 working days after reception of the application and proceeds with the assessment.
- A.3.1.6 The application and accompanying documents are reviewed by the COR Lead Auditor to completeness of the application within 15 working days.
- A.3.1.7 When information is missing, the COR Lead Auditor informs the applicant of the necessary additional documentation and indicates that no further processing of the application shall take place until all required information is submitted.
- A.3.1.8 The applicant is required to respond to the clarification questions and document requests within 30 working days or the file shall be closed.
- A.3.1.9 When the COR Lead Auditor determines that the information is complete, the process of document and record review starts.
A.3.2 Document and record review
- A.3.2.1 The COR Lead Auditor or other designated CFIA staff shall review all relevant documents and records supplied by the applicant to evaluate its system, as documented, for conformity with the ISO/IEC 17011 requirements as referenced in the SFCR and the additional requirements specified in the COR operating manual.
- A.3.2.2 Upon completion of the document review, the COR Lead Auditor prepares document review report which indicates any nonconformities (NCs) and opportunities for improvement (OFIs) with the requirements and requests for further information, if necessary.
- A.3.2.3 In some cases, when the number of NCs are very high the COR Lead Auditor may decide to cease the document review and shall notify the applicant accordingly.
- A.3.2.4 The document review report is sent to the applicant, with a request to take necessary actions to conform to the applicable requirements.
- A.3.2.5 The CVB has 60 working days to submit evidence of corrective actions for all NCs.
- A.3.2.6 On receipt of the corrective actions COR Lead Auditor reviews the submission and assesses the extent to which the required NCs have been addressed.
- A.3.2.7 Once the COR Lead Auditor assesses that all the amended documents confirm with ISO/IEC 17011 and COR operating manual an on-site assessment (audit) shall be arranged.
A.3.3 On-site assessment
- A.3.3.1 The on-site assessment is conducted by the COR audit team.
- A.3.3.2 The COR audit team prepares and sends to the applicant all the information and documentation needed for the on-site assessment including audit plan and assessment criteria in advance.
- A.3.3.3 During the on-site assessment, the COR audit team shall require access to the following information: organizational setup, personnel, management system documents, internal audit reports, management review reports, accreditation procedures, accreditation records, certification bodies' files, personnel files for the purpose of verifying training records and performance monitoring. The applicant shall ensure this information is available and easily retrievable whether in hard copy or electronic form. Any findings during the on-site audit shall be classified as a NC or an OFI.
- A.3.3.4 The on-site audit shall end with an exit interview where the NCs and OFI shall be presented and discussed.
- A.3.3.5 The applicant shall be given time period of 30 working days to provide corrective actions on the identified NCs from the date of the exit interview. OFIs are to be addressed at the applicant's time frame.
- A.3.3.6 The COR audit team shall draft out an assessment report which shall include the findings from the on-site assessment.
- A.3.3.7 The draft assessment report shall be provided to the applicant within 30 working days after the on-site audit is completed. It includes the NCs, if any, and OFIs.
- A.3.3.8 The applicant reviews the report content, verifies the accuracy of the facts and submits any corrections to CFIA within a time frame specified by the CFIA.
- A.3.3.9 The final assessment report shall be reviewed and approved by the CFIA National Manager responsible for COR and a copy shall be sent to the applicant.
- A.3.3.10 COR Lead Auditor shall verify the implementation of the corrective actions submitted by the applicant before providing recommendation to the CFIA for decision on entering into an agreement with the applicant.
- A.3.3.11 In case the CFIA decides not to enter into an agreement with the applicant, the CFIA shall notify the applicant of its right to request a decision review of the designation process by the CFIA management.
A.3.4 Witness audit
- A.3.4.1 The witness audit shall be conducted as a means of verifying that the applicant is satisfactorily implementing its procedures.
- A.3.4.2 During the witness audit, the COR audit team shall examine the applicant's auditor's preparation for the audit and the implementation of the applicant's auditing procedures.
- A.3.4.3 The COR audit team and the applicant auditor(s) shall have a closing meeting to discuss any findings identified during the audit in a manner that they are understood and acknowledged by the applicant.
- A.3.4.4 The COR audit team shall draft a witness audit report that shall be shared with the applicant within 30 working days after the witness audit is completed.
- A.3.4.5 The applicant shall review the report content, verify the accuracy of the facts and submit any corrections to CFIA.
- A.3.4.6 The final witness report shall be approved by the CFIA National Manager and a copy shall be sent to the applicant without undue delay.
- A.3.4.7 The witness report shall be issued separately both during the initial and reassessment year and the surveillance years.
A.3.5 Decision review process
- A.3.5.1 Any applicant organization can request a review of a designation decision. The request shall be made within 30 working days of notification of the decision in writing to CFIA Executive Director responsible for the Canada Organic Regime. The Executive Director shall review the request and notify the applicant of his decision.
- A.3.5.2 The decision of the Executive Director in this regard shall be final.
A.3.6 Agreement signature
- A.3.6.1 Based on the results from the final assessment report, the CFIA shall enter into an agreement with the applicant.
- A.3.6.2 The agreement between the CFIA and the CVB expires on the fifth year and shall require renewal every 5 years following the initial assessment.
A.4 Monitoring and surveillance of CVB
A.4.1 The CFIA shall monitor the ongoing compliance of the CVB with the regulations and the Agreement.
A.4.2 The CVB shall submit an annual update report in accordance with A.10.
A.4.3 Under the COR agreement cycle as outlined in section A.9, the CFIA shall conduct CVB on-site surveillance assessment in the first, third and fifth year. In the second and fourth year there shall be a document review based on the CVB annual report.
A.4.4 During the 5 year agreement cycle, the COR audit team shall conduct 1 witness audit per CVB every year following A.3.4. The COR team may choose to witness a CVB audit at the CB office, CVB witness audit or CVB verification audit.
A.4.5 The surveillance assessments are conducted following a review of the annual report. During the surveillance assessment the COR audit team shall review the compliance with the agreement and certain elements of the COR.
A.4.6 Following the surveillance assessment the COR audit team shall draft out a surveillance report which shall include the findings from the on-site assessment.
A.4.7 The draft surveillance report shall be provided to the CVB within 30 working days after the on-site audit is completed. It includes the NCs, if any, and OFIs.
A.4.8 The CVB reviews the report content, verifies the accuracy of the facts and submits any corrections to CFIA within a time frame specified by the CFIA.
A.4.9 The final surveillance report shall be reviewed and approved by the CFIA National Manager responsible for COR and a copy shall be sent to the CVB.
A.4.10 If any NCs are found during the surveillance the CVB shall be given up to 30 working days from the exit interview to respond.
A.4.11 At any time and upon its own discretion, the CFIA may carry out additional assessments for any NCs with the agreement, regulations and CFIA requirements. The CFIA shall advise the CVB of this possibility.
A.4.12 The CFIA may conduct unscheduled assessments or visits to the CVB as a result of valid complaints or changes to the regulations.
A.5 Renewal of the agreement between CFIA and CVB
A.5.1 All the CVBs shall undergo full reassessment on the fifth year of the agreement signature. The procedure for the agreement renewal is the same as the one for initial CVB assessment outlined in A.3 and includes resubmission of all required documentation, on-site assessment and witness audit.
A.5.2 The CVBs shall submit all required documentation 8 months prior to the agreement expiration date to allow for the reassessment to be completed in timely manner.
A.6 Breach of the agreement, suspension and early termination
A.6.1 The CVB understands that its failure to meet any of the terms of the agreement is considered by the CFIA a breach of the Agreement and as a result, the CFIA could take actions including suspension measures and termination of the agreement.
A.6.2 If during monitoring of the compliance with the agreement, the CFIA notices NCs, it shall issue a report to the CVB outlining the NCs and the period in which a corrective action plan should be submitted to the CFIA for approval. Upon receipt of the report, the CVB signs it.
A.6.3 Following the report, the CFIA shall provide a notice to the CVB which specifies the period within which the CVB shall have to provide to the CFIA a corrective action plan with defined timeline for approval.
A.6.4 If the CVB fails to provide a corrective action plan within the specified period in the notice, the CFIA shall suspend the CVB.
A.6.5 If the CVB provides to the CFIA a corrective action plan within the specified period in the notice, the CFIA shall verify the adequacy of the proposed corrective action and approve it if it is satisfactory to the CFIA.
A.6.6 If the CFIA is not satisfied with the adequacy of the proposed corrective action, the CFIA shall send a notice for revision to the CVB to revise their corrective action plan with a specified period.
A.6.7 The CVB shall submit to the CFIA a revised corrective action plan for approval within the period specified in the notice for revision.
A.6.8 The CFIA shall review and approve the revised correction action plan if it is satisfactory. The process is on-going until corrective action plan is approved by the CFIA.
A.6.9 The CVB shall implement the corrective action plan as approved by the CFIA. The CVB could be subject to suspension if failing to do so.
A.6.10 The CFIA shall verify the implementation of the corrective action plan and submit a report to the CVB.
A.6.11 If the CVB fails to implement the corrective action plan within the prescribed time period to the CFIA's satisfaction, the CFIA shall submit a report to the CVB outlining the NCs.
A.6.12 The CFIA shall send a notice of suspension to the CVB, outlining the grounds for suspension, the required corrective measures and the period within which those measures shall be implemented to avoid termination of the agreement.
A.6.13 During the suspension period, the CVB is not authorized to accept new applications for accreditation and conduct initial assessment and reassessment for accreditation however; the CVB shall continue conducting its surveillance activities as planned.
A.6.14 Furthermore, the CVB shall provide to the CFIA an updated list of the CBs under their supervision and a list of pending applications for accreditation within 5 working days after receipt of the notice of suspension.
A.6.15 The CFIA may lift the suspension after it has conducted an assessment to verify that the CVB has implemented the corrective measures within the period specified in the notice of suspension.
A.6.16 The CFIA may, at its sole discretion, terminate the agreement in the event the CVB does not implement the corrective measures within the period specified in the notice of suspension.
A.6.17 Each party may decide to terminate the agreement for any other reasons. That party shall give to the other party a minimum of 60 working days' notice prior to the termination of the agreement.
A.6.18 In the event that the agreement is terminated, CFIA shall notify the affected CBs and give them some time to find another assessed CVB to continue their accreditation.
A.7 Complaints against CVBs
A.7.1 Every complaint concerning a CVB's accreditation activities shall be submitted to the COR Lead Auditor in writing and accompanied by justifying evidence or documents.
A.7.2 The COR Lead Auditor shall acknowledge the complaint within 5 working days in writing.
A.7.3 The COR Lead Auditor shall designate a person from CFIA to follow-up on the complaint or decide to follow-up on the complaint.
A.7.4 The designated person shall gather all required information and prepare a report which is submitted to the COR Lead Auditor at the conclusion of the process.
A.7.5 The complainant shall be informed that the CFIA took appropriate action to correct the situation. However, the nature of the action shall remain confidential. If no further issues arise, the CFIA shall close the file.
A.7.6 The CFIA maintains the record of each complaint, the corrective and preventive actions taken and the effectiveness of such action.
A.8 CVB documents required for initial assessment
A.8.1 CVB documents to be submitted along with the application for designation
- A.8.1.1 The corporate charter
- A.8.1.2 Any government act, regulation or decree that gives the CVB the legal authority to accredit prior to becoming a CVB under the COR.
- A.8.1.3 The corporate structure showing graphically and quantitatively relations of control by shareholders, companies or other groups of the organization.
- A.8.1.4 The general bylaws.
- A.8.1.5 A list of directors, comprising:
- A.8.1.5.1 Members of the board of directors (including specific function, duration of mandate, and affiliation).
- A.8.1.5.2 Board members of a sponsoring organization (if applicable).
- A.8.1.6 The addresses of all locations where the CVB does business and summary of activities from each location.
- A.8.1.7 A copy of the compliance mark (body's name such as it appears on accreditation certificates and any property rights related to it prior to becoming a CVB under the COR).
- A.8.1.8 A copy of the liability insurance for directors and employees.
A.8.2 Description of CVB decision making structures
- A.8.2.1 A description of individuals or Internal bodies making decisions covering:
- A.8.2.1.1 Assessment of applicants
- A.8.2.1.2 Accreditation of applicants
- A.8.2.1.3 Appeals
- A.8.2.1.4 Complaints
- A.8.2.2 A description of sharing of responsibilities between head office and affiliates (if applicable).
- A.8.2.3 An organization chart related to the general administration of the program including names of persons occupying managerial positions in both head office and affiliates (when it applies).
A.8.3 Information on CVB's operations
- A.8.3.1 A complete list of all CBs including the name and address of every one to which the CVB has granted accreditation for production of organic products prior to becoming a CVB under the COR.
- A.8.3.2 A copy of the board of Director's latest annual report to members or stockholders.
A.8.4 CVB standards, policies and technical procedures (quality manual)
- A.8.4.1 The quality manual related to the accreditation program.
- A.8.4.2 The templates for assessment questionnaires used by auditors.
- A.8.4.3 The templates for audit reports.
- A.8.4.4 Lists of documents included in the file on each CB having requested accreditation.
- A.8.4.5 Copy of IAF evaluation of the CVB or other third party assessment against ISO/IEC 17011 standard (if available).
A.8.5 CVB's human resources management
- A.8.5.1 A complete list of employees associated with the CVB to work on COR accreditation including the status and position held by each one.
- A.8.5.2 A copy of the standard contract with these employees.
- A.8.5.3 The selection criteria for persons making accreditation decisions or in charge of overseeing those who make them.
- A.8.5.4 The name of person or list of the members of the internal body committee, etc. assigned either to make accreditation decisions or to oversee those who make them (with their experience or specific training).
- A.8.5.5 The selection criteria for assessors and experts.
- A.8.5.6 A copy of the standard contract with contract assessors.
- A.8.5.7 A complete list of contract assessors (including their training and years of experience, their commercial or financial affiliation).
- A.8.5.8 A copy of the standard contract used with any subcontractors (if applicable).
A.8.6 Information, material and forms forwarded to accreditation applicants
- A.8.6.1 A detailed CVB fee schedule for the various services offered (to be available for review during the on-site assessment by CFIA).
- A.8.6.2 Copies of information documents about the COR accreditation program within the CVB that would be provided to potential clients.
- A.8.6.3 A copy of the application forms to be filled out by applicants.
- A.8.6.4 A list of documents that shall be supplied to the CVB by an applicant's CB.
A.8.7 Documents concerning rights and obligations of designated CVBs
- A.8.7.1 A copy of the contract (template) between the CVB and the CB, to be signed, when the CB is granted accreditation by CVB prior to becoming a CVB under the COR.
- A.8.7.2 An example of an accreditation certificate issued by the CVB prior to becoming a CVB under the COR.
A.9 Canada Organic Regime assessment cycle
The following table outlines the types of audits that the CFIA shall conduct of its designated CVBs in the first 5 years after the COR became effective. This same sequence of events shall be logically extended to cover those years subsequent to those shown below.
| Applicant assessment year | First year | Second year | Third year | Fourth year | Fifth year |
|---|---|---|---|---|---|
| Initial AssessmentTable Note 1 | On-site surveillanceTable Note 1 | Document review | On-site surveillanceTable Note 1 | Document review | Re-assessmentTable Note 1 |
| One witness audit | One witness audit | One witness audit | One witness audit | One witness audit | One witness audit |
A.10 Annual information from the designated CVBs
This section lists those documents or information that the designated CVBs shall submit annually to the CFIA as a part of the on-going monitoring of the designated CVBs.
The information shall be submitted before the end of the first quarter of the calendar year and shall cover the previous 12 months.
- A.10.1 A list of all CBs under their supervision including those transferred from other CVBs with information concerning their corporate entity, name, business addresses; and a description of the certification services that the CBs undertake.
- A.10.2 The number of CBs who have applied for assessment
- A.10.3 Total number of surveillance audits
- A.10.4 Total number of witness audits
- A.10.5 Total number of verification audits
- A.10.6 Total number of reassessment audits
- A.10.7 Total number of complaints under COR
- A.10.8 Total number of appeals under COR
- A.10.9 Copy of the internal audit report
- A.10.10 Copy of the Management's review
- A.10.11 Information on the personnel change
- A.10.12 Information on policy change
Part B Accreditation of certification bodies (CB)
Participation in the Canada Organic Regime (COR) accreditation program is not intended to prevent certification bodies (CB) from carrying out other business activities, especially those involving the certification of products not covered by the scope of the regulations.
Operations resulting from these other activities should neither constitute an infringement nor result in conflicts of interest with the certification program accredited by the CFIA.
B.1 Objective
This section outlines the CB accreditation requirements and the requirements for the CVB assessing and monitoring the CB responsible for the certification under the COR.
The CVB shall ensure that CB seeking CFIA accreditation to offer certification under COR are compliant with the requirements of part 13 of the SFCR including ISO/IEC 17065 and the requirements of this manual.
Accreditation is obtained as a result of a rigorous process. The applicant shall undergo an assessment conducted by CVB in accordance with ISO/IEC 17011 to verify the CB's compliance with ISO/IEC 17065, the requirements of the COR Operating Manual, the CFIA directives and memos.
On the recommendation of the CVB, the CFIA accredits the applicant CB. The CFIA shall provide the accredited CB with an accreditation number. The accreditation number granted by the CFIA to a CB means the latter, being a competent, responsible and qualified party has the financial and organizational capacity to manage a certification program that shall result in consistent and credible certification decisions.
B.2 Initial CB accreditation
B.2.1 Application By CB and document review by CVB
- B.2.1.1 An applicant applying for accreditation under the COR shall submit an application form to a designated CVB.
- B.2.1.2 In addition to the application form, the applicant shall provide all supporting documents as listed in section B.11 and any additional documents deemed essential for the assessment as requested by the CVB.
- B.2.1.3 The CVB shall send acknowledgement of receipt to the applicant CB within 10 working days, shall notify the CFIA about the application, and proceeds with the assessment.
- B.2.1.4 The applicant shall pay the application fees determined by the CVB.
- B.2.1.5 The CVB shall conduct a resource review (as of ISO/IEC 17011 section 7.3) to assess the CVB's ability to carry out the assessment.
- B.2.1.6 The CVB shall prepare for the assessment (as per section 7.5 of ISO/IEC 17011).
- B.2.1.7 The CVB shall conduct the document review against the COR requirements (including ISO/IEC 17065, part 13 of the SFCR, COR Manual, CFIA Directives and Memos) and shall communicate the findings from the document review to the CB. It shall include the identification of any nonconformities (NCs) and/or information requests.
- B.2.1.8 The CVB shall require the applicant CB to provide response for all NCs and information requests. The CVB shall determine which NCs shall be resolved before proceeding with an on-site assessment.
- B.2.1.9 The CVB may communicate with the applicant CB or an independent source, in order to obtain any other information needed to examine the application.
B.2.2 On-site assessment of CB
- B.2.2.1 The CVB shall conduct the assessment following the requirements outlined in section 7.6 of ISO/IEC 17011.
- B.2.2.2 The CVB shall analyze all relevant information and evidence to determine the competence and extent of conformity of the applicant with the COR requirements, including compliance with part 13 of the SFCR, ISO 17065, COR Operating Manual, the CFIA directives and memos.
- B.2.2.3 The CVB shall select an assessment team that shall proceed with an on-site assessment covering the applicant's certification activities. The CVB may assign 1 or more members of its personnel and may also retain the services of external auditors or technical experts or both.
- B.2.2.4 The appointed CVB auditor(s) should not have been employed by a CB in a position within a 2 year period from the appointment.
- B.2.2.5 The criteria relative to a CVB auditor's competence, qualifications and experience shall include, among others:
- B.2.2.5.1 Knowledge and understanding of the COR's requirements including accreditation criteria and procedures
- B.2.2.5.2 Knowledge of the Canadian Organic Standards and generally accepted experience such as practical experience in production, processing, inspection or certification management would be a major asset relative to conformity assessments
- B.2.2.5.3 Knowledge of auditing principles, procedures and methods including interviewing techniques and an ability to draft reports in compliance with ISO/IEC 19011 requirements
- B.2.2.6 The names of the assigned auditors shall be communicated to the CB, who may, based on serious concerns, object to the assignment of any auditor mentioned. In light of the reasons stated by the CB, the CVB may appoint another auditor or retain the one initially selected.
- B.2.2.7 In circumstances where the applicant CB has multiple offices, the CVB shall visit the main office and will select to visit the CB satellite offices based on the key activities (ISO 17011 7.4.5) concerning the certification process.
- B.2.2.8 The CVB shall send to the applicant CB the information, documentation and relevant instructions needed to conduct witness audit and verification audits, as well as an estimate of expenses pertaining to this visit.
- B.2.2.9 The CVB auditor(s) shall begin every visit with an opening meeting with the applicant's representatives and at a minimum explain the audit objectives relative to accreditation criteria, review the audit plan, and confirm the scope of the evaluation.
- B.2.2.10 The CVB auditor(s) shall conduct interviews with the relevant CB personnel including managers, employees and contractors, as required.
- B.2.2.11 The CVB auditor(s) shall carry out rigorous examination of a sampling of the applicant CB certification files. The CVB auditor(s) shall randomly select the files to be included in the sample, with consideration given to the CB's certification and geographical scope. The examination of files shall ensure that:
- B.2.2.11.1 The documentation found in an operator's file (for example, signed contracts, initial and updated production/preparation plans, letter of good standing in case of CB change, inspection reports, certification, certification decision and other correspondence, approved inputs, labels, copies of certificates) are complete and up to date
- B.2.2.11.2 The inspection reports include sufficient information and evidence needed to make a proper certification decision
- B.2.2.11.3 The certification decision made by the applicant CB is in line with the evaluation of the operator's production/preparation plan and the results from the inspection report
- B.2.2.11.4 The applicant CB has verified the implementation of all corrective measures that were requested from the operator
- B.2.2.11.5 The applicant CB is operating in accordance with the relevant sections of the ISO/IEC 17065
- B.2.2.12 The CVB auditor(s) shall base the quantity and selection of files to be examined on the following sampling rules:
- B.2.2.12.1 The CVB auditor(s) shall carry out file reviews according to the table below.
| Number of active operators registered with the CB under COR | Number of files to be reviewed |
|---|---|
| Less than 5 | All files are subject to a full review |
| Less than 100 | Minimum of 5 full reviews Table note 2 (the number could be increased at the discretion of the CVB) |
| 101 to 240 | Minimum of 10 Table note 3 files, 5 of which must be full reviews |
| 241 to 400 | Minimum of 12 files, 6 of which must be full reviews |
| 401 to 1000 | Minimum of 15 files, 7 of which must be full reviews |
| More than 1000 | Minimum of 20 files, 10 of which must be full reviews |
- B.2.2.13 The CVB auditor(s) shall verify the competence of the personnel involved in the certification activities of the CB, within the framework of the positions they occupy. The CVB auditor shall review these employees' competence, training and education and shall conduct interviews with some of them.
- B.2.2.14 The CVB auditor shall conduct at least 1 witness audit as a means of verifying that the applicant CB is implementing its procedures satisfactorily.
- B.2.2.15 The CVB auditor shall, during the witness audit(s), observe the VO preparation for the inspection and his/her adherence to the CB's inspection procedures.
- B.2.2.16 For CBs that do not yet have clients in the organic sector, the CVB shall conduct a witness audit as soon as the CB has an operator.
- B.2.2.17 The CVB auditor shall present the findings from the on-site visit and the witness audit to the CB in a format determined by the CVB.
- B.2.2.18 The CVB shall establish its own reporting procedures in compliance with ISO/IEC 17011.
- B.2.2.19 The CVB shall allow the applicant CB a time period of 30 working days from receiving the CVB report to submit the specific actions taken or planned to be taken in order to resolve the identified NCs.
- B.2.2.20 The CVB shall evaluate whether the responses and action taken by the applicant CB to resolve any NC appears sufficient and effective as outlined in sections 7.6.8 and 7.6.9 of the ISO/IEC 17011.
- B.2.2.21 If the CVB determines that the provided information is not sufficient or adequate, further information may be requested and/or additional assessment activities may be conducted.
- B.2.2.22 CFIA may accompany the CVB assessment team to observe the accreditation process.
B.2.3 CVB recommends accreditation to the CFIA
- B.2.3.1 The CVB shall decide to either recommend or not recommend the accreditation of the applicant CB to the CFIA.
- B.2.3.2 The CVB shall only recommend the applicant CB for accreditation if all identified NCs have been adequately addressed by the applicant and when the CVB is confident that the applicant CB has fulfilled the requirements for accreditation.
- B.2.3.3 The CVB shall send to the CFIA the recommendation decision in writing and shall provide to the CFIA a copy of the CVB evaluation report on the applicant CB and any other relevant information to support the accreditation recommendation.
- B.2.3.4 The CFIA shall review the CVB recommendation. If the CFIA decides to confirm the CVB recommendation, it shall grant accreditation to the applicant.
- B.2.3.5 If the CVB recommends to not accredit the applicant CB, the CVB shall send a notice to the applicant CB by registered mail or email (with confirmation of receipt from the applicant), stating the reason for the decision. The applicant CB has the right to request that the CFIA review the CVB decision within 30 working days after receipt of the notice.
- B.2.3.6 If the CFIA does not agree with the CVB recommendation the CFIA shall grant accreditation to the CB.
- B.2.3.7 The CFIA shall inform the applicant CB and the CVB on its decision to accredit or not to accredit.
B.2.4 CFIA grants the accreditation
- B.2.4.1 The CFIA shall inform the applicant CB and the CVB of the accreditation decision made by the CFIA by issuing an accreditation letter.
- B.2.4.2 The CFIA shall grant accreditation valid for 5 years beginning on the date the accreditation number is granted by the CFIA.
- B.2.4.3 The CB shall be re-assessed, recommended by a CVB and accredited by the CFIA for another 5 years before the end of the accreditation cycle in order to have its accreditation renewed once this period has ended.
B.2.5 Requirements for granting accreditation number to the CB
- B.2.5.1 CFIA shall assign the CB an accreditation number allowing it to provide certification services under the COR and shall issue a letter which specifies the certification scope and geographical scope for which the accreditation is granted.
- B.2.5.2 The CFIA shall assign an accreditation number no later than 14 working days after the accreditation decision.
- B.2.5.3 The CB shall keep the same accreditation number that they have received originally as long as their accreditation remains valid.
B.3 Monitoring and surveillance of a CB
B.3.1 The CVBs shall be responsible for on- going monitoring of the accredited CB in compliance with the COR requirements including part 13 of the SFCR, ISO 17065, COR Operating Manual, the CFIA directives and memos.
B.3.2 The CVBs shall document their procedures and plans for carrying out periodic on-site assessments and other surveillance activities to verify that the accredited CB continue to fulfill the COR requirements. In cases of extenuating circumstances such as natural disasters, political unrest, the CVBs may modify their surveillance activities.
B.3.3 The surveillance visits shall target the verification of specific CB's certification program elements.
B.3.4 After the initial accreditation, the CVB shall conduct an on-site surveillance of the CB within 12 months of the initial accreditation date.
B.3.5 Prior to conducting an on-site assessment the CVB shall request from the CB updated information, on a date specified by the CVB and review it. The information from the CB shall include the following:
- B.3.5.1 Changes in the CB information
- B.3.5.2 Major changes to the CB policies, procedures and protocols
- B.3.5.3 Information on complaints and appeals
- B.3.5.4 The most recent internal audit report
- B.3.5.5 The most recent management review report
- B.3.5.6 All reported misuses of the Canada organic logo received by the CB
- B.3.5.7 All changes in the CB certification personnel that are critical to the operation of its certification activities
- B.3.5.8 Complete list of certified operations in the COR including name, address and phone number of the certified entity, the type of the operation certified (crops, livestock, processing, wild crop). If provided via a directory on the Internet, it is acceptable provide the URL to the directory instead
- B.3.5.9 Complete list of operations certified to the terms of Canada's organic equivalence arrangements including name, address and phone number of the certified entity, the scope of certification and their locations. If provided through a directory on the Internet, it is acceptable to provide the URL to the directory instead
B.3.6 Over the length of the accreditation cycle, for each surveillance visit, the CVB auditor shall examine a number of files, proportional to the number of the active operators registered with the CB, and based on the numbers shown in the table below.
| Number of active operators registered with the CB under COR | Number of files to be reviewed |
|---|---|
| Less than 100 | Minimum of 5 full reviewsTable note 4 (the number could be increased at the discretion of the CVB) |
| 101 to 240 | Minimum of 6 files, 5 of which must be full reviewsTable note 5 |
| 241 to 400 | Minimum of 8 files, 6 of which must be full reviewsTable note 5 |
| 401 to 1000 | Minimum of 10 files, 7 of which must be full reviewsTable note 5 |
| More than 1000 | Minimum of 12 files, 8 of which must be full reviewsTable note 5 |
B.3.7 The CVB shall, over the length of the accreditation cycle, conduct witness audits according to the table below as a means of verifying that the accredited CB implements its procedures as written.
| Number of active operators registered with the CB under COR | Total number of witness audits over the CB accreditation cycle |
|---|---|
| Less than 100 | 1 witness audit |
| 101 to 240 | 2 witness audits |
| 241 to 400 | 3 witness audits |
| 401 to 1000 | 4 witness audits |
| More than 1000 | 5 witness audits |
B.3.8 The CVB shall, over the length of the CB accreditation cycle, conduct verification audits according to the table below to verify the information included in the operators' files.
| Number of active operators registered with the CB under COR | Total verification audits over the CB accreditation cycle |
|---|---|
| Less than 100 | 1 verification audit |
| 101 to 1000 | 2 verification audits |
| More than 1000 | 3 verification audits |
B.3.9 The CVB shall choose the operator for the verification and witness audits. CVB should take into consideration the CB schedule for the upcoming on-site inspections when selecting the operator for witness audits.
B.3.10 During the verification audit, the CVB auditor shall verify, among other matters that:
- B.3.10.1 The operator has on hand a copy of the CB's certification requirements, as well as any requests for corrective measures submitted to the operator by the CB from the previous CB inspection.
- B.3.10.2 The certified products/ activities are within the scope of part 13 of the SFCR.
- B.3.10.3 The inspection report adequately describes the production system.
- B.3.10.4 The inspection process was able to adequately reveal points of noncompliance with the standard
B.3.11 The CVB auditor shall record the findings from the on-site visit, the witness audit and the results from the verification audits. The format of each report shall be determined by the CVB.
B.3.12 The CVB shall allow the CB 30 working days from receiving the CVB report to submit the specific actions taken or planned to be taken in order to resolve the identified NCs.
B.3.13 When the CVB is satisfied with the CBs corrective actions provided within the specified time frame, the CVB shall inform the CB of the results from the surveillance activities by issuing a letter indicating that the CB continues to maintain its compliance with the COR. The CVB shall send a copy of this letter to the CFIA.
B.3.14 The CVB shall apply one of the enforcement actions as specified in B.7 in the event that NCs have not been addressed satisfactorily within the 30 working days from the receipt of the CVB report.
B.3.15 The CVB may conduct additional assessments as a result of complaints or significant changes that have affected CB operations at the expense of the CB, at any time during the accreditation cycle, or upon its own initiative.
B.3.16 The CFIA may conduct an unscheduled assessment of an accredited CB at any time during the accreditation cycle as a result of complaints or concerns, or at its own discretion.
B.3.17 At the request of the CFIA, the CVBs shall compile, review and submit the annual information from the CB using the CFIA template. The following information will be included in the CB annual report:
- B.3.17.1 A list of operators in a spreadsheet, including:
- legal name of the operator
- address of the operator
- type of operation (primary, processing, packaging and labelling, attestation) and
- generic names of the certified products
- B.3.17.2 Number of new certificates (both organic products and packaging)
- B.3.17.3 Number of annual inspections and un-announced inspections
- B.3.17.4 Number of nonconformities and samples taken
- B.3.17.5 Number of complaints
B.3.18 At any time during the accreditation cycle the CVB may accept request from the CB to extend the scope of accreditation. The CVB shall have documented procedure to address such request. The CVB shall recommend to the CFIA scope expansion when the CVB is confident that the CB has fulfilled the requirements for accreditation.
- B.3.18.1 Geographical scope and Accreditation category addition
- B.3.18.1.1 The CVB shall review the CB request/application and supporting documents confirming availability and the training of the verification officers (VO).
- B.3.18.1.2 The CVB does not need to conduct a witness audit (WA) if there are no operators at the time of the application, however the CVB shall confirm the expertise and knowledge of the CB personnel involved in the certification process.
- B.3.18.1.3 The CVB shall send to the CFIA a recommendation letter to extend the CB's geographical scope/accreditation category along with the results from the review.
- B.3.18.1.4 The CFIA will make the final decision and will amend the accreditation letter accordingly.
- B.3.18.1.5 During the next planned CB office audit, the CVB auditor shall verify how the new scope/category is managed by the CB (interview with key personnel, review of the first operator's file, etc.).
- B.3.18.1.6 The CVB shall schedule a WA in the new geographical location/accreditation category as soon as the CB has an operator.
- B.3.18.2 Addition of aquaculture standard
- B.3.18.2.1 The CVB shall review the CB request/application and supporting documents.
- B.3.18.2.2 The CVB shall be satisfied with the expertise of the CB staff that is involved in the aquaculture standard.
- B.3.18.2.3 The CVB is not required to conduct a WA or have a meeting with the CB if:
- B.3.18.2.3.1 the CB is already accredited for aquaculture standard outside COR
- B.3.18.2.3.2 the CB is already accredited for CAN/CGSB-32.310 to certify wild collection and they want to add CAN/CGSB-32.312 for seaweed
- B.3.18.2.3.3 the CB is already accredited for CAN/CGSB-32.310 to certify processed products and want to add CAN/CGSB-32.312 for processing of aquaculture products
- B.3.18.2.4 In all the other cases (not covered by B.3.18.2.3) the CVB shall schedule and conduct a WA on the new standard as soon as the CB has an operator.
- B.3.18.2.5 The CVB shall send to the CFIA a recommendation letter to add the aquaculture standard along with the results from the review.
- B.3.18.2.6 The CFIA will make the final decision and will amend the accreditation letter accordingly.
- B.3.18.2.7 During the next planned CB office audit, the CVB auditor shall verify how this new scope is managed by the CB (interview with key personnel, review of the first operator's file, etc.).
B.4 Reassessments of a CB
B.4.1 The CB shall apply for reassessment according to the CVB's own procedure to allow the CVB to complete all assessment activities before the accreditation expires.
B.4.2 In the event of reassessment the CVB shall follow the requirements for initial assessment outlined in sections B.2.1 and B.2.2.
B.4.3 Following the reassessment, the CVB shall follow the requirements for accreditation recommendation outlined in section B 2.3.
B.4.4 The CB shall continue to be responsible for providing access to records, files and other related documentation to the CVB and the CFIA during reassessment and continued accreditation oversight activities.
B.5 Appeals of CFIA accreditation decision by a CB
B.5.1 Any applicant CB has the right to request that the CFIA review the accreditation decision. The appeal against the decision shall be made within 30 days of notification of that decision pursuant to SFCR section 362.
B.5.2 The appeal shall be filed in writing along with all the necessary supporting documents.
B.5.3 The CFIA shall give the final decision on the appeal. The decision of the CFIA in this regard shall be final.
B.6 Appeals of CVB recommendation decision of a CB
B.6.1 The CVB shall document their own appeal policy and procedure to deal with appeals against final recommendations made by the CVB to the CFIA and also against specific CVB decisions.
B.6.2 The CVB policies shall address appeals of the following decisions as minimum:
- B.6.2.1 Decision whether to proceed with a visit
- B.6.2.2 Decision regarding any additional visit
- B.6.2.3 Decision to terminate an evaluation process
- B.6.2.4 Decision whether to recommend the reduction of accreditation scope to CFIA
B.7 Reduction of scope, suspension and cancellation of a CB
B.7.1 The CVB shall follow their documented procedures for identification and management of NCs.
B.7.2 The CVB shall apply 1 or more of the following enforcement actions in an event the NCs are not addressed within the specified time frame:
- B.7.2.1 Recommend to the CFIA to reduce the accreditation scope
- B.7.2.2 Submit to the CFIA report for grounds for suspension of the CB
B.7.3 The CVB shall submit a report for grounds for suspension in any of the following cases:
- B.7.3.1 the CB has failed to effectively implement the corrective actions or where the assessment reveals that the CB has failed to effectively implement the corrective actions. related to conditions that have previously been considered fulfilled
- B.7.3.2 if the CB does not have any operators after 2 consecutive surveillance assessments in an accreditation cycle
B.7.4 The CVB shall provide the written report to the CB and the CFIA describing the grounds for suspension and the CVB shall allow the CB 15 working days to take corrective action as per paragraph 364(2)(a) of the SFCR.
B.7.5 The CVB may grant 1 extension period upon written request from the CB supported by justification as per subsection 364(3) of the SFCR. The length of the extension period is at the discretion of the CVB but should not be longer than 60 working days.
B.7.6 In the case when the CVB is satisfied that the CB has resolved the issues that were grounds for suspension within the time allowed, the CVB shall notify the CFIA and recommend continuation of accreditation or re-accreditation.
B.7.7 In the case when the CVB is not satisfied with the CB's corrective actions, the CVB shall recommend suspension to the CFIA.
B.7.8 The CFIA shall issue the notice of suspension and the date on which it takes effect.
B.7.9 The CB has 15 days after the day the accreditation was suspended to submit their list of operators as per subsection 364(5) of the SFCR.
B.7.10 The CB has 30 days after the day the accreditation was suspended to take corrective action as per paragraph 365(1)(a) of the SFCR.
B.7.11 The CB shall submit their corrective action to the CVB for review and assessment. The CVB shall determine the acceptability of the corrective action plan within 10 working days.
B.7.12 In the case when the CVB is satisfied with the CB's corrective actions, the CVB shall recommend lifting the suspension to the CFIA as per subsection 364(6) of the SFCR.
B.7.13 In the case when the CVB is not satisfied with the CB's corrective actions or the CB did not submit corrective actions within the time allowed, the CVB shall recommend to the CFIA cancellation of accreditation as per subsection 365(1) of the SFCR.
B.7.14 The CFIA shall issue a notice in writing on the grounds for cancellation to the CB and give the CB opportunity to be heard as per subsection 365(2) of the SFCR. The CFIA shall allow 20 working days to the CB for the opportunity to be heard.
B.8 Complaints against CB
B.8.1 The CVB shall document its policies and procedures that outline how complaints related to accredited CB and their operators are handled by the CVB.
B.8.2 The CVB shall acknowledge receipt of any complaint received from the CFIA within 5 working days.
B.8.3 The CVB shall begin the investigation of the complaint as per its own procedures, or forward the complaint to the appropriate CB for investigation if the complaint is on a certified organic product, producer or CB personnel.
B.8.4 When forwarding complaints to the appropriate CB, the CVB shall ensure the complainant's confidentiality is maintained.
B.8.5 Within 20 working days, the CVB shall inform the CFIA of the following:
- the status of the complaint
- what actions have or will be taken to resolve the complaint
- the expected timeline for resolution of the complaint
- requests for additional information as required
B.8.6 The CVB shall endeavour to close all complaints received in a timely manner. Upon closure of the complaint the CVB shall provide the CFIA with the following:
- confirmation that the CVB considers the complaint adequately closed
- a summary of the actions the CVB/CB took to close the complaint
- any important follow-up information (for example, unannounced audit to verify, grounds for suspension, etc.)
B.8.7 The CFIA shall inform the CVB that the complaint is considered closed once the CFIA has reviewed the information provided and determines that no additional information is required.
B.8.8 In case that the complaints cannot be resolved between the CB and the CVB, the CFIA is the final step to hear the issue.
B.9 Records maintained by the CVB
B.9.1 The CVB shall maintain records on the CB they recommended for accreditation to demonstrate that the requirements for accreditation, including competence, have been effectively fulfilled. The records to be maintained include:
- B.9.1.1 general features of the CB, including corporate entity, name, addresses, legal status and human and technical resources
- B.9.1.2 general information concerning the CB such as its activities, its relationship in a larger corporate entity if any, and addresses of all its physical location(s) to be covered by the scope of accreditation
- B.9.1.3 clearly defined scope of accreditation
- B.9.1.4 a contract to fulfill the requirements for accreditation and the other obligations of the CB, including submitting all necessary documentation requested in section B.11
- B.9.1.5 a description of the conformity assessment services that the CB undertakes, and a list of standards, methods, or procedures for which the CB seeks accreditation, including limits of capability where applicable
- B.9.1.6 a copy (on paper or in electronic form) of the quality manual of the CB, and relevant associated documents and records (refer to section B.11)
B.10 CVB agreement with the CB
B.10.1 The CVB shall prepare and implement surveillance agreement (contract) between the CB and CVB that outlines the rights and duties of the CB and the CVB which shall be signed by the CB and the CVB.
B.10.2 The CVB shall provide a sample of this agreement as part of the application package provided to the CB.
B.11 CB documents required for accreditation application (except the cases when a CB changes their CVB)
This section lists those documents or information that the applicant CB is to submit to the CVB as part of its initial and reassessment application as a CB.
B.11.1 Documents pertaining to the CB
- B.11.1.1 The corporate charter
- B.11.1.2 The corporate structure showing graphically and quantitatively relations of control by shareholders, companies or other groups for the organization
- B.11.1.3 The general by laws
- B.11.1.4 A list of directors, comprising:
- members of the board of directors (including specific function, duration of mandate, and affiliation)
- board members of a sponsoring organization (if applicable)
- B.11.1.5 The addresses of all locations where the firm does business and summary of activities from each location
- B.11.1.6 A copy of the compliance mark (body's name such as it appears on the label or certified product) and any property rights related to it
- B.11.1.7 In the case of CB already accredited by an official organization (for example, another accreditation body), a copy of the accreditation certificate for the CB from the other organization
B.11.2 Description of decision making structures
- B.11.2.1 A description of individuals or internal bodies making decisions covering:
- product certification
- appeals
- brand name control (certifying body's name and logo)
- along with their mandate, their procedures, and the manner in which they are designated
- B.11.2.2 A description of sharing of responsibilities between head office and affiliates (if applicable)
- B.11.2.3 An organization chart related to the general administration of the program including names of persons occupying managerial positions in both Head Office and Affiliates (if applicable)
B.11.3 Information on CB's operations
- B.11.3.1 Copy of the latest annual financial statements, including balance sheet, revenues and expenses
- B.11.3.2 List of countries, provinces or states in which the body is carrying out certification activities
- B.11.3.3 Complete list of all firms including the name and address of each 1 to which the body has granted a compliance certificate, in the 1 or more fields for which it has applied for accreditation:
- a compliance certificate for the certified products
- a certificate of recognition for any inputs or services
- B.11.3.4 Copy of the Board of Director's latest annual report to members or stockholders
B.11.4 Standards, policies and technical procedures (quality manual)
- B.11.4.1 The quality manual related to the certification program
- B.11.4.2 Templates of inspection questionnaires used by VO
- B.11.4.3 Templates of inspection reports
- B.11.4.4 List of documents included in the file for each operator having requested certification
B.11.5 CB human resources management
- B.11.5.1 A complete list of certification employees including the status and position held by each one.
- B.11.5.2 A copy of the standard contract with certification employees
- B.11.5.3 The selection criteria for persons making certification decisions and persons in charge of overseeing people who make certification decisions
- B.11.5.4 The name of persons or list of the members of the internal body (committee, etc.) assigned either to make certification decisions or to oversee those who make them (with their experience or specific training)
- B.11.5.5 The selection criteria for the VOs
- B.11.5.6 Copy of standard contract between the CB and VO
- B.11.5.7 Complete list of VOs (including their training and years of experience, their commercial or financial affiliation)
- B.11.5.8 A copy of the standard contract used with any other type of subcontractors (if applicable)
B.11.6 Information material and forms forwarded to an applicant
- B.11.6.1 A detailed fee schedule for the certification services offered
- B.11.6.2 Copies of information documents about the certification program
- B.11.6.3 Copy of the application forms to be filled by applicants
- B.11.6.4 Copies of production or preparation compliance plan forms to be filled annually by applicants
B.11.7 Documents concerning rights and obligations of certified operators
- B.11.7.1 Contract(s) to be signed by certification applicants, regulating the use of marks of compliance (licenses)
- B.11.7.2 Copy of the certificate issued by the CB in accordance with the COR and equivalency arrangement (if applicable)
- B.11.7.3 Electronic copy of a label using the name of the CB and Canada Organic Logo
B.12 Requirements when a CB changes CVB under the COR
This section is applicable to the situation where a CFIA accredited CB chooses to change their CVB.
B.12.1 Requirements on CB
- B.12.1.1 The CB shall submit an application form to another designated CVB and notify the current CVB of the decision to change
- B.12.1.2 The CB shall provide all supporting documents as requested by the receiving CVB
- B.12.1.3 The CB shall pay the application fees determined by the receiving CVB
- B.12.1.4 The CB shall cease use of the previous Accreditation letter and destroy it.
B.12.2 Requirements on sending CVB
- B.12.2.1 The CVB shall inform the CFIA immediately when a CB notifies them of their intention to change CVBs
- B.12.2.2 The CVB shall provide to the CFIA the results from the last CB audit (copy of the most recent audit reports, associated NCs status and any outstanding issues)
B.12.3 Requirements on receiving CVB
- B.12.3.1 The CVB shall send acknowledgement of receipt to the applicant CB within 10 working days and shall notify the CFIA immediately about the application
- B.12.3.2 After CFIA reviews the documentation provided by the sending CVB, and after discussion between the receiving CVB and the CFIA, the receiving CVB shall:
- B.12.3.2.1 Accept the compliance status of the CB with further oversight activities as agreed by the CFIA and the receiving CVB
- B.12.3.2.2 Send a recommendation letter to the CFIA to confirm that the CVB will take over the monitoring of the CB and to request amendment to the current CB accreditation letter
- B.12.3.2.3 Take over the monitoring of the CB from the point in the accreditation cycle established by the sending CVB
B.12.4 Requirements on CFIA
- B.12.4.1 The CFIA shall verify the CB status with the sending CVB and shall request the CVB provide to the CFIA, the results from the last CB audit (copy of the most recent audit reports, associated NCs status and any outstanding issues)
- B.12.4.2 The CFIA shall review the documentation provided by the sending CVB and shall discuss the CB status with the receiving CVB to ensure that the change is smooth and without negative impact. The CFIA shall discuss with the receiving CVB the outstanding requirements of the accreditation cycle
- B.12.4.3 The CFIA shall issue a revised Accreditation letter to the CB after receiving a recommendation letter from the receiving CVB
- B.12.4.4 The CFIA shall change only the name of the CVB on the revised Accreditation letter
- B.12.4.5 The CFIA shall request the CB to return to the CFIA the previous Accreditation letter once they receive the revised letter
B.13 Requirements for voluntary withdrawal of a CFIA accredited CB under the COR
This section is to address the situation when a CB accredited by CFIA wishes to withdraw voluntarily its CFIA accreditation under COR.
B.13.1 Requirements on CB
- B.13.1.1 The CB shall send a written notice to the CVB that monitors the CB activities under COR
- B.13.1.2 The CB shall submit to the CVB the list of holders of certifications and a list of pending applications for certification as per sub section 364(5) from part 13 of the SFCR
- B.13.1.3 The CB shall notify the holders of certifications within 3 months after the CB has sent the written notice to the CVB to give them sufficient time to find another certification body
- B.13.1.4 The CB shall surrender the CFIA accreditation letter before it expires
B.13.2 Requirements on CVB
- B.13.2.1 The CVB shall acknowledge the receipt of the CB notification within 10 working days
- B.13.2.2 The CVB shall notify the CFIA immediately when a CB has indicated its intention to withdraw its accreditation
- B.13.2.3 The CVB shall submit a recommendation letter to the CFIA for decision on the withdrawal of accreditation
- B.13.2.4 The CVB shall ensure that any reference to the COR on the CB's website and on CB promotional materials is removed
B.13.3 Requirements on CFIA
- B.13.3.1 The CFIA shall review the CVB recommendation letter
- B.13.3.2 The CFIA shall send, upon recommendation from the CVB, a notice of cancellation to the CB as per subsection 365(1) of the SFCR
- B.13.3.3 The CFIA shall remove the CB name from the list of the CFIA accredited certification bodies on the date of the accreditation cancellation.
B.14 Requirements when a CB goes out of business
This section is to address the situation when a CB accredited by CFIA goes out of business.
The term "going out of business" is broad and includes a spectrum of financial states of a CB. One end of the spectrum could include a CB that is experiencing financial difficulty, but is still operational and able to meet their financial obligations, but may become insolvent in the future. The other end of the spectrum could include a CB that has declared bankruptcy. Also included in between the 2 ends of the spectrum might be CBs that are insolvent but not yet bankrupt and who may file a proposal to avoid bankruptcy.
B.14.1 Requirements on CB
- B.14.1.1 The CB shall notify immediately its CVB in cases where it plans to stop certifying organic products or it may become unable to continue to certify organic products.
- B.14.1.2 The CB shall provide to the CVB the list of holders of certifications and the list of pending applications for certification as per subsection 364(5) of part 13 of the SFCR.
- B.14.1.3 The CB shall not accept new applications for certification during this period of financial uncertainty but shall make every effort to complete the certification process of the existing applicants.
B.14.2 Requirements on CVB
- B.14.2.1 The CVB shall request the CB to provide the list of holders of certifications and the list of pending applications for certification as per subsection 364(5) of part 13 of the SFCR when the CVB becomes aware that the CB is planning to stop certifying organic products or it may become unable to continue to certify organic products.
- B.14.2.2 The CVB shall notify the CB of its inability to accept new applications if the CVB has determined that the CB is planning to stop certifying organic products or it may become unable to continue to certify organic products.
- B.14.2.3 The CVB shall monitor the certification activities of this CB to ensure that it makes every effort to complete any ongoing certifications.
- B.14.2.4 The CVB shall work with the CFIA and the CB to inform the operators at the appropriate time.
- B.14.2.5 The CVB shall send a recommendation for accreditation cancellation to the CFIA if the CB ceases to conduct business.
B.14.3 Requirements on CFIA
- B.14.3.1 The CFIA shall cancel the accreditation of the CB under section 365 of part 13 of the SFCR in cases when the CB ceases to conduct business.
- B.14.3.2 The CFIA and the CVB shall work together to ensure that all the proper actions are taken as per part 13 of the SFCR.
B.14.4 Requirements on the operators
- B.14.4.1 It the responsibility of the operator to apply to a new CB within the time prescribed in subsection 344(3) of part 13 of the SFCR and follow the steps as described in section C.2.5 of the COR Manual if they wish to continue their certification.
B.15 Requirements when a CVB can conduct remote audits at CBs
This section outlines the criteria that should be considered by a CVB when scheduling and conducting a remote audit at a CFIA accredited CB.
The "remote audit" is an audit that uses electronic means to remotely obtain evidence to determine the extent of conformity to the audit criteria.
A remote audit can be "partial" when only some parts of the audit (for example operator file checks) are conducted remotely or "full" when the whole audit (all checks) is completed remotely.
- All audits conducted by CVBs may be performed remotely in case of:
- climactic or socio-economic conditions are unfavorable and/or make them impossible, (for example for safety reasons: pandemics, country at war, conflict or instability, extreme weather condition)Footnote 6
- In normal conditions B.15.1.1 applies
B.15.1 Eligibility
B15.1.1 The CVBs may conduct the following audits remotely:
- surveillance audit
- additional assessment - as a result of a complaint
- follow-up audit - to verify implementation of a corrective action following a NC
B15.1.2 The CVBs shall not conduct the following audits remotely:
- initial audit
- reassessment audit
- witness audit
- verification audit
B.15.2 Requirements on CVB
B.15.2.1 The CVB may consider conducting a remote audit if the CB has the capacity and technical resources (for example good internet connection) to be assessed remotely with secure access to the CB intranet for reviewing documentation in any of the following possible situations:
- the CB has a good certification system in the previous 2 years (for example in relation to the number of NCs, complaints, appeals)
- CB system is not complex (for example number of operators, type of certification, grower group)
- additional CB offices with no key activities
- documentation review and interviews are sufficient to make a decision on a minor change in current accreditation
B.15.2.2 The CVB shall define the means of on-line communication ( for example provide information on the audit techniques) and make a compatibility test of the communication platform before confirming a remote audit.
B.15.2.3 The CVB shall determine whether the remote audit is acceptable and possible to conduct and will notify the CB in writing.
B.15.2.4 The CVB shall develop and share with the CB an audit plan with specific timetables (for the CB and the CVB). The CVB shall confirm with the CB whether this plan is acceptable.
B.15.2.5 The CVB shall specify whether the CB must be online or whether the auditor works alone (for example documentary analysis, preparation for the closing meeting) in the audit plan or during the opening meeting.
B.15.2.6 The CVB shall not conduct more than 2 consecutive full remote audits at a specific CB.
B.15.2.7 The CVB shall not schedule a remote audit at a CB with recurring and numerous issues (for example noncompliance, notice of intention to suspend, complaints, appeals).
Part C Certification of organic product and CB requirements
C.1 Objective
This section provides guidance on the certification process including application for certification, evaluation, decision on certification and continuation of the certification under the Canada Organic Regime (COR). It also provides requirements on the CB. The CVB shall verify how the CB meets these requirements during every initial, surveillance or reassessment audit conducted by the CVB.
C.2 Procedures for certification under COR
C.2.1 Application for initial certification
C.2.1.1 The CFIA accredited CB shall ensure that person seeking certification of their products or packaging and labelling activities make an application as defined by the CB, in accordance with Division 4 of part 13 of the Safe Food for Canadian Regulations (SFCR).
C.2.1.2 The CB shall require that the applicant provide all the relevant documents and information deemed essential to the assessment as described in subsection 344(2) of the SFCR. In addition, the application shall include the name(s) of CBs to which prior applications for certification were submitted by the applicant within the previous years (under COR or other schemes such as USDA NOP, EU, JAS etc.), including all details pertaining to processing the application, and the resulting decision.
C.2.1.3 The CB shall ensure that the applicant pays the fees for certification according to the CB's contract for services and in accordance with the CB's fee schedule.
C.2.1.4 The CB shall verify whether the applicant holds other types of certifications - packaging and labelling certificate and/or attestation of compliance.
C.2.1.5 The CB shall verify that the applicant does not hold a valid Canadian Organic Standards certificate for an identical/same product, issued by another CFIA accredited CB and if the product is certified as organic under another organic system (such as USDA NOP, EU, JAS etc.).
C.2.1.6 The CB shall verify the submitted documentation for completeness and to determine if it has the competence and capacity to perform the certification activity.
C.2.2 Application evaluation
C.2.2.1 The CB shall document its procedure for its evaluation activities. The CB shall evaluate the application against the requirements set out in CAN/CGSB-32.310, CAN/CGSB-32.311 and CAN/CGSB-32.312 as applicable to the nature of the product and production system.
C.2.2.2 The CB shall verify that the substances and the materials used in the production of organic products comply with CAN/CGSB 32.311 and CAN/CGSB-32.312 as applicable to the nature of the product and production system. The CB must maintain a procedure and documentation to support its determination about the status of input compliance.
C.2.2.3 The CB can determine input compliance with CAN/CGSB 32.311 or CAN/CGSB-32.312 as applicable to the nature of the product and production system by contacting the supplier/formulator/manufacturer to obtain full disclosure of the ingredients in the input material and the processes used to produce the ingredients and the input material.
C.2.2.4 The CB may consult with another CFIA accredited CB that has already evaluated a specified input material and, accept that CB's assessment of the input's compliance with CAN/CGSB 32.311 or CAN/CGSB-32.312.
C.2.2.5 The CB may consult with a third party organisation that is accredited under ISO 17065 to conduct input evaluation.
C.2.2.6 The CB shall take responsibility for all input evaluations including those activities outsourced to a third party.
C.2.2.7 The CB shall periodically confirm that input product formulations and processes have not changed. This shall generally be annually, but where a longer interval can be justified, must be at least once every 5 years.
C.2.2.8 The CB shall file a complaint to the CVB or directly to the CFIA if the CB has evidence that another CB has approved an ineligible input. If a CB becomes aware that another CB has rejected an input that they have accepted, the first CB can also submit a complaint.
- C.2.2.8.1 The CBs and CVB(s) shall come to collective decision on the status of the input within 60 working days. The CVBs may consult technical experts with knowledge in inputs in order to reach an unbiased decision. Any costs/fees associated with this would be the responsibility of the complaining CB(s) if the complaint is found to be unfounded and by the CB(s) that improperly approved the input if the complaint is upheld. In case the CVBs and CBs cannot reach an agreement they can file a complaint with the CFIA.
C.2.2.9 The CB shall schedule an on-site inspection of the applicant to determine compliance with CAN/CGSB-32.310, CAN/CGSB-32.311 and/or CAN/CGSB-32.312, as applicable to the nature of the product and production system.
C.2.2.10 The timing of the on-site inspection shall take place during the production season in cases involving producer operations. This period begins as soon as the operations subject to inspection (seeding, tapping, etc.) begin and ends with the packaging or placing in containers for storage of products to be certified.
C.2.2.11 In cases involving processing operations, on-site inspections may be carried out any time during the year. On the other hand, for separated production (that is, when both certifiable and non-certifiable products are manufactured at the same facility), the inspection shall be carried out at the time when the products that are targeted for certification are being processed. If the CB determines it is not possible to conduct the inspection while organic product is being processed, the CB shall record the reason(s) supporting this determination. The CB shall then arrange for the inspection to be conducted at a time when the facilities and activities that demonstrate compliance or capacity to comply can be assessed.
C.2.2.12 The CB shall ensure that the applicant is contacted to arrange the logistics of the on-site inspection.
C 2.2.13 The CB shall record the VO selected to conduct on-site inspection. It is recommended that verification officers not be scheduled to inspect the same operation for more than 3 consecutive years. If there are extenuating circumstances and the VO inspects the same operation for more than 3 consecutive years, the justification must be recorded.
C.2.2.14 The CB shall allow the applicant to refuse the selected VO in case of conflict of interest.
C.2.2.15 The CB shall ensure that the assigned VO conducts an opening meeting with a representative of the applicant to confirm the inspection objectives, scope and criteria.
C.2.2.16 The CB shall ensure that all production and processing operations (for example, fields, crops, plants, livestock, buildings, facilities and vehicles), including packaging and labelling and any subcontracted activities upon which an operator relies to produce and/or prepare each product included within its application are inspected by the assigned VO who will verify that the standards are fully applied and correspond to the submitted production or preparation specifications.
C.2.2.17 The CB shall ensure that the land, premises and equipment not included in the scope of certification are identified and included in the application. The VO must verify the list is complete and correct including, at a minimum, the following: crop areas or harvesting zones; harvest storage locations; preparation, processing and packaging sites, a complete list of phytosanitary products used by the operator. Further, the VOs must verify the operator maintains a record of application dates and locations for phytosanitary products.
C.2.2.18 The CB shall verify that prohibited substances have not been, and are not being, applied to the operation.
C.2.2.19 The CB shall ensure that the VO conducts a review of record keeping, to verify that the organic plan submitted to the CB accurately reflects the operation and is in compliance with CAN/CGSB-32.310 or CAN/CGSB-32.312 as applicable to the operation and nature of production. Records to be verified shall include records related to production (for example, inventory, sales, purchases, organic certificates for each ingredient received) and to management (for example, accounting, complaints); as well as appropriate product packaging and labelling.
C.2.2.20 The CB shall ensure that the VO identifies and inspects areas of risk (for example, potential contamination from neighbouring farm, flooding, undeclared split production).
C.2.2.21 The CB shall ensure that for producers, the VO obtains an estimate of the potential yield for the coming year, as well as an audit of the balance in the quantities produced and sold over the previous period, and including amounts still in inventory during this same period.
C.2.2.22 The CB shall ensure that for applicants performing operations related to food preparation (processing and/or packaging), the VO calculates the input/output balance for acquired commodities and for the corresponding inputs included in the products sold and on inventory. The calculation sample shall include more than 1 run of the product and at least 1 prominent commodity. Where there is no organic product available during first inspection, the operator's system for input/output shall be verified.
C.2.2.23 The VO shall strive to review different commodity at each inspection, if applicable. However, if justified by the VO, additional commodity(ies) may be included in this calculation. This justification shall be recorded in the inspection report.
C.2.2.24 As per 4.4 of CAN/CGSB 32.310, the CB shall ensure that the VO performs products/ingredients trace back audits to verify that the integrity of the organic product is maintained. Products and/or their ingredient components must be traceable to the operators own production locations (fields/plots) or suppliers as applicable to the product and nature of the operation. Trace back audits will be checked based on conventional products to ensure the operator has established an effective traceability system where there is no organic product available during first inspection.
C.2.2.25 The CB shall ensure that the VO interviews people knowledgeable within the operation at the time of inspection.
C.2.2.26 The CB shall ensure that at the end of the visit the VO conducts a closing meeting to:
- inform the operator's representative of inspection results as well as findings made concerning the compliance with certification requirements, without any corrective action request from the VO
- provide opportunity for the operator to confirm the accuracy of information collected during the inspection, by signing the exit report (paper copy or electronic copy)
- provide a summary of this review in writing to the operator
C.2.2.27 The VO shall submit to the CB a report mentioning verification results and findings as to the conformity with all certification requirements, and including the following data as a minimum:
- date, time and duration of inspection
- names of interviewees (main representatives involved with the inspection)
- identification of land and premises visited on the production/handling site
- types of documentation audits performed (in/out balance sheet, yields/sales, trace back exercise, label review etc.)
- inspection results
- list of findings identified by the VO
C.2.3 Review
C.2.3.1 The CB shall inform the operator of all NCs with reference to the applicable clause from the standard and shall require from the operator to respond to the NC report issued by the Certification Body within 30 working days of its receipt. The response shall either provide evidence of completion of corrective action(s) taken to address each NC or present a plan with milestones as to how each NC will be addressed. This plan shall include a completion date not exceeding 90 working days from receipt of the NCs. The CB shall accept times greater than those stated for the closure of a NC as long as they are justified and documented.
C.2.3.2 In the case where the operator requests and the CB grants a one-time extension (SFCR paragraph 349 (2) (b)), the period of extension may extend beyond 90 working days from receipt of the NCs as long as the times are justified and documented.
C.2.3.3 The CB shall ensure that corrective actions aiming to address all nonconformities have been implemented by the operator by conducting an on-site visit or other appropriate forms of verification.
C.2.3.4 The CB shall inform the applicant that at any point within the certification cycle, preceding the CB's decision, the applicant may request that the processing of its application be stopped. The applicant shall be informed that they are liable for the costs of services provided up to the time of withdrawal of its application. In such case, the CB shall not issue a decision regarding the products that were subject of the certification request.
C.2.4 Certification decision
C.2.4.1 If a CB has reason to believe that an applicant for initial certification has willfully made a false statement regarding its production system and operations related to the products included in the application, the CB may deny certification, without issuing a notification of noncompliance.
C.2.4.2 The CB shall issue a written notice of denial of certification to any applicant to whom it denies certification, either because operations resulting in the products included in the application are still noncompliant with requirements or simply because the applicant did not respond to the notification of noncompliance. This notice shall state the reason(s) for denial and the applicant's right to:
- file an appeal of the denial with the CB
- reapply for certification to any accredited CB, including the one who denied certification
C.2.4.3 The decision to certify a product and/or packaging and labelling activities shall be taken if the CB determines that all procedures and activities contained in the organic plan are in compliance with the SFCR requirements and that the applicant is able to operate in accordance with its plan and after the correction of all nonconformities. This decision is valid until the results of the next annual evaluation are known and a new decision is made or unless the CB is made aware of information to cause the CB to act (for example, suspension or cancellation). This information can come from an external source or from the CB's own efforts.
C.2.4.4 The CB shall provide the applicant with a certificate that confirms the certification of the organic product and/or certification of packaging and labelling activities of the organic product. In accordance with ISO 17065 7.7.1, these certificates shall include the following:
- The wording "Pursuant to part 13 of the Safe Food for Canadians Regulations (SFCR)…"
- The name, address, and contact information of the certification body (as appears on the CFIA web site)
- Certification number (as issued by the CB, it will vary by CB )
- The name and address of the holder of the certificate, whereby the holder is the legal name of the person(s) who produced/processed/packaged/labelled/traded the product and that had applied for certification. A "person" means "an individual, a corporation, an association, or an organization recognized as a legal entity"
- When applicable, the certificate or document should also include the name of the holder who commonly does business under, or the name which the holder is commonly known by in the marketplace. All other non-legal names/business names on the certificate in addition to the legal name shall be clearly referred to as "doing business as" or "DBA". The certificate cannot bear the names of multiple legal entities. A parent company and any of their subsidiary companies are separate legal entities
- A list of the certified products, which shall be identified by their specific product name and any trademarks under which they are marketed. Product names on certificates should coincide with label/shipping bill/import-export documentation
- in the case of a multi-ingredient food commodity, whether at least 70% of its contents are organic products or whether at least 95% of its contents are organic products
- The standards under which each product or product type is certified (CAN/CGSB 32.310 or CAN/CGSB 32.312)
- The wording "Certified in accordance with the terms of the U.S.-Canada Organic Equivalency Arrangement" (when applicable)
- The applicable type of certification:
- Crop production
- Livestock production
- Livestock feed
- Specialized production
- apiculture
- maple products
- mushroom production
- crops grown in structures or containers
- wild crops
- sprouts, shoots and microgreens
- insects
- Processed products (for example, processed agricultural and/or livestock products)
- Aquaculture products including aquaponic products
- Trade of organic products (for example, importer, exporter, distributor)
- Group certification
- the date on which the certification was initially granted
- the date of last inspection
- the date by which the operator shall submit application for subsequent annual inspection as per subsection 346 (1) of the SFCR
- the location (address, town, province/state, country) of all production units covered by this certification ( includes but is not limited to: all production, processing, harvest, and storage sites). (not applicable for grower group members)
- The following wording "This certification remains valid unless suspended or cancelled by the (INSERT THE NAME OF THE CB) pursuant to part 13 of the Safe Food for Canadians Regulations (SFCR)"
- Date, name and signature of CB representative
The Organic Product Certificate will only be issued once the organic product meets the requirements of the Canadian organic standards. In case of first time applicants, if no organic product is available, the CB can issue a letter confirming that the applicant has an organic system plan in place for organic products as specified in the organic system plan. The letter must contain the following wording "This letter cannot be used for marketing organic products".
C.2.4.5 Packaging and labelling activities certificate shall include the period of validity, the type(s) of organic products to which the certification applies.
C.2.4.6 The certification of a product, once issued, shall remain valid unless suspended or cancelled by the CB according to the requirements of the SFCR.
C.2.4.7 The CB shall follow subsection 350(3) of the SFCR requirements for cancellation in the case of voluntary withdrawal by the operator.
C.2.5 Procedure for continuation of certification
C.2.5.1 The CB shall document the procedures to verify annually that the SFCR requirements for certification continue to be met by the holder of certificate.
C.2.5.2 The CB shall require the holder of the certificate to submit the information specified in subsection 346(1) of the SFCR once every 12-month period, which begins on the day on which the certificate is issued. The CB shall require the holder of the certificate to submit their intention to maintain certification in writing no later than the date that is 6 months prior to the end of that period and the completed recertification documentation in a time frame specified by the CB and as appropriate to the nature of the operation.
C.2.5.3 The CB shall proceed with an on-site inspection to verify compliance with the applicable requirements as outlined in C.2.2 when the holder of certificate submits all information requested by the CB. There shall be no more than 2 consecutive years without an inspection of the organic product being processed (applicable only for processed products).
C.2.5.4 The CB shall ensure that the VO verifies on-site that any changes in the organic standards and the CB requirements have been effectively implemented by the operator.
C.2.5.5 The CB shall ensure that the VO verifies on-site that previously submitted corrective actions have been, and remain, fully implemented.
C.2.5.6 The CB shall ensure that the onsite inspection/verification is completed prior to the end of the 12 month period from the day the certificate was granted as per SFCR subsection 346 (2).
C.2.5.7 The CB shall verify that all the requirements for certification are met resulting either in continuation of the certification or initiation of suspension and cancellation.
C.2.5.8 The CB shall make its certification decision for continued certification as outlined in C.2.4.
C.2.6 Additional inspections
C.2.6.1 In addition to the annual inspections the CB shall plan and conduct unannounced inspections representing 5% of the CB's clients to which CB grants certificates for products and/or packaging and labelling and/or attestation of compliance under the COR. Exceptions to the 5% rule are listed in the table below.
| Number of operators registered with the CB under COR | Number of unannounced inspections |
|---|---|
| 1 to 4 | 1 unannounced inspection per CB accreditation cycle |
| 5 to 32 | 1 unannounced inspection per year |
| 33 or more | Unannounced inspections conducted as per section C 2.6.1 of the COR Operating manual |
C.2.6.2 The CB shall document the procedure covering the frequency and selection criteria for these unannounced on-site inspections. In cases where it is not possible to conduct an unannounced inspection (for example, for reasons related to site access or any other factors supported by a justification), advance notice may be given providing that this notice period does not allow time to cover up noncompliances that might exist. In any case the notice shall be not more than 24 hours. The CB shall document the reasons for any advance notice.
C.2.6.3 Unannounced inspections shall be limited in scope and shall cover only certain aspects of the operation. Every year the operators for unannounced inspections shall be randomly selected based on risk, and/or as a result of a complaint or investigation. The CB may disclose to the operator the reason for the unannounced or additional inspection.
C.2.6.4 The CB shall consider the following examples when developing risk-based criteria for unannounced inspections (list is not exhaustive):
- Type of operator (producer, processor, packager)
- New or experienced operator (categories for number of years' experience can be used)
- Size and complexity of operation (for example, total area under production, complexity of value chain)
- Type and value of product (for example, short supply, high price, susceptibility to disease or pests, ratio of price to quality)
- Number of parcels of land or animal units under transition
- Local geography (for example, lay of land, buffer areas, water supply, presence of neighbours and types of neighbouring land uses, nearby spray operations)
- Only organic, split operation or parallel production
- Total quantity of products produced and/or processed
- Rapid increase in production versus stable production levels
- Compliance history (nonconformities in previous inspections)
- Complaints received
- Suspicion of fraud
- Quality of information (information supplied in certification process)
- Economic fraud risk (multiple contracted suppliers, group certification)
- Detection of chemical residues or signs of prohibited substances
- Irregularities in mass balance calculations and traceability records
- Number of new suppliers
- Number of changes to management team
- Change in ownership
- Brand names (number produced under the operation, processor not using own name)
- Higher risk animal production systems
C.2.6.5 The CBs shall apply a checklist of risk based criteria when evaluating the risk to which the integrity of organic products can be compromised. The CB shall define individual scores that can be applied to each risk criteria. These scores should be added to calculate a total score for each operator. Based on the scores, the CB will determine which operators are selected for unannounced inspections.
C.2.6.6 In addition to the scheduled 5% unannounced inspections, the CB shall comply with any requests from the CFIA or the CVB to conduct additional inspections (announced or unannounced) when the compliance of the operation is in doubt or for other valid reasons.
C.2.7 Sampling and testing
C.2.7.1 The CBs shall develop sampling and testing procedure incorporating the following sampling criteria:
- Type of product (for example, susceptibility to disease or pests or usually high uses of pesticides in conventional systems)
- Local geography (for example, lay of land, buffer areas, water supply, presence of neighbours and types of neighbouring land uses, nearby spray operations)
- Complaints or information previously received regarding the potential use of prohibited substances
- Detection of chemical residues
- Signs of prohibited substances
C.2.7.2 The CB shall ensure that the verification officers (VOs) are trained on how to sample, label, and store products under proper chain of custody until samples are submitted for testing.
C.2.7.3 The CB shall ensure that the VOs are provided with the proper sampling equipment at the time of the inspection as per specific sampling protocols provided by the laboratory.
C.2.7.4 The CB should consider referring to Codex Alimentarius Commission (Codex) for information on recommended methods of sampling for the determination of chemical residues as guidance.
C.2.7.5 The CB shall be responsible for maintaining the chain of custody of samples prior to submission to the laboratories.
C.2.7.6 The CB shall have a legally binding contract with laboratory(s) that hold current accreditation to ISO/IEC 17025: 2017 - General Requirements for the Competence of Testing and Calibration of Laboratories, and for which the scope of accreditation allows for the testing of the specific substance in food.
C.2.7.7 CFIA accredited CBs must maintain records to demonstrate that they conduct chemical residue sampling and testing of their operators products and follow up on chemical residue results that were forwarded to them for action. CBs must consider the guidance document "Directive 14-01: Procedure for follow-up on positive chemical residue results in organic products" and "Chemical residues and organic production" when following up on any chemical residue positive result.
C.2.7.8 The CB shall require pre-harvest or post-harvest testing of any organic product to be sold, labelled or represented as being in compliance with the requirements of the CAN/CGSB-32.310, CAN/CGSB-32.311 and/or CAN/CGSB-32.312 as applicable to the nature of the product and production system when there is a reason to suspect that the organic product has come into contact with a prohibited substance, method or ingredient in the production and handling of organic products.
C.2.7.9 The CB may sample and test as part of an investigation of a complaint concerning the use of or contamination with prohibited substance. Intentional use of prohibited substances by an operator shall result in the CB initiating the suspension and cancellation process as per part 13 of the SFCR.
C.2.7.10 The CB shall investigate if there is suspicion that an organic product has been contaminated by or commingled with genetically engineered (GE) materials. The investigation may include sampling and testing for GE presence.
C.2.8 Suspension, cancellation and reinstatement
C.2.8.1 The CB shall suspend and cancel a certification as per part 13 of the SFCR.
C.2.8.2 The CB shall report to its CVB all suspensions, change of a CB by an operator, and cancellations it issues on the 25th of each month, in case such decisions are made, or shall be provided as defined by the CVB. All reports shall include the name of the holder of certificate, the date of issue and the reason for the action.
C.2.8.3 The CB shall not grant certification to an applicant who had its certification previously cancelled and whose name appears on the CFIA published list of cancelled organic certifications unless the applicant has submitted an application for certification to a CFIA accredited CB as per section C.2, and has completed the evaluation process and closed all the nonconformities.
C.2.8.4 The CB shall submit to the CFIA a request for removing the name of the holder of certificate from the list of cancelled holders of certificates posted on the CFIA web site.
C.2.8.5 The CB shall proceed with granting a certification after receiving conformation from the CFIA that the operator is removed from the CFIA list.
C.2.8.6 The Organic Production Systems: General Principles and Management Standards (CAN/CGSB-32.310) require that the Standard be fully applied on a production unit at least 12 months before the harvested product is considered organic for these food commodities (per 5.1.1 and 7.2.8). These 12 months must be under the oversight of a CFIA accredited certification body (CB).
C.2.8.7 A cancelled operator who wishes to be reinstated must apply as a new applicant as per section 344 of the SFCR. CFIA interprets the requirements as follows:
- C.2.8.7.1 the production unit (the applicant) must be under CB oversight for at least 12 consecutive months prior to the harvest of a product in order for it to be covered by the organic certificate (issued by the CB at the end of the 12 months).
- C.2.8.7.2 an applicant cannot market products harvested during or prior to the start of the 12 months of oversight as organic.
- C.2.8.7.3 For the requirements of the standard to be met, the oversight is based on the time of harvest, and not the expected time of sale.
C.3 Timing of sale or distribution of certified product
C.3.1 When certification is requested, the CB shall ensure that the applicant does not offer for sale any product "represented as organic" or bearing the word organic or its derivatives until the applicant receives the certificate from the CB.
C.4 Complaint and appeal
C.4.1 The CB shall document procedures to ensure that it deals with the complaints and appeal by applicant, certificate holder or other party pertaining to certification in accordance with the requirements specified in ISO 17065.
C.4.2 The CB documented procedures shall deal with, as a minimum:
- C.4.2.1 appeal related to certification decisions
- C.4.2.2 complaints from holders of certificates regarding the CB's program application
- C.4.2.3 complaints from outside persons or organizations about the CB's operation
C 4.3 The CB shall communicate the next steps to the certificate holder in case the holder is not satisfied with the CB appeal process. The certificate holder can submit a complaint against the CB to the CVB responsible for the oversight of the CB.
C.5 Issues regarding implementation of the standard
C.5.1 The CB shall notify all of its certificate holders of any amendments to the regulations or the standards within 2 months after their publication.
C.5.2 The CB shall allow a period of up to 12 months after the publication date of an amendment to CAN/CGSB-32.310, CAN/CGSB-32.311 and CAN/CGSB-32.312 for applicants to come into compliance with any changes to the requirements.
C 5.3 Some of the revisions in the standards may require more than 12 months to implement, such as barn renovations to comply with new flock sizes, exit spaces and natural lighting in poultry installations. When applicable, any period longer than 12 months is specified within the standards.
C 5.4 The CB shall update application documentation, training materials, certification procedures and checklists to reflect the most recent versions of the Canadian Organic Standards.
C.5.5 If an interpretation of an applicable standard is required by the CB or a certificate holder at any point during certification activities, it can be sought from the Standards Interpretation Committee (SIC).
C.5.6 It is likely that the need for interpretation requests to the SIC will occur during a certification cycle of a certificate holder by a CB. In such cases, where both parties agree there is need for interpretation or clarification and the interpretation request is submitted by the CB, the issue that is the subject of the request will be set aside by the CB (for example, the nonconformity will be placed on hold) until the response from the SIC is returned.
C.5.7 In these cases, between the time when the interpretation request to the SIC is submitted and the response from the committee returned, any certification work affected by the interpretation shall proceed as normal, up to the issuance of certification documents.
C.5.8 When the response from the SIC is received, the outstanding issue shall be revisited and appropriate actions taken by the CB or the operator or both, as required.
C.5.9 If changes are required by the certificate holder to comply with the interpretation of the SIC, the CB shall not suspend or withdraw any certification it has issued that is affected by this interpretation as long as the operator has made the required changes in a time frame that is no less than the time permitted for any other nonconformance issued by the CB.
C.5.10 In cases where the CB and the certificate holder do not agree that the issue needs an interpretation, the CB shall rely on part 1.4 of CAN/CGSB-32.310 or part 1.4 of CAN/CGSB-32.312 when interpreting the issue. The certificate holder is still able to make a complaint to the CVB about the CB and/or ask the SIC for an interpretation and request a reconsideration of the issue at a later date.
C.5.11 The CB shall adhere to the interpretations of the Canadian Organic Standards (CAN/CGSB 32.310, CAN/CGSB 32.311, and CAN/CGSB 32.312) provided by the SIC and considered official by the CFIA, to achieve uniform and consistent application of the rules to all operators through various CBs.
C.5.12 The CBs shall inform their certificate holders about these interpretations.
C.6 Use of licenses, certificates and marks of conformity
C.6.1 The CB shall ensure that all certified products are labelled in accordance with the SFCR.
C.6.2 The CB shall have procedures to monitor the holders of certificates using its certification mark and its name and marketing organic products to detect any improper reference to the Canada Organic Regime or fraudulent use of the CB name and certificates.
C.6.3 The CB shall have written rules authorizing the use of its mark (including the recognition of product labels on which it shall be displayed) and is responsible for delivering the organic certificates.
C.6.4 The CB shall have written procedures for dealing with abusive use, false statements regarding a product's certification or the incorrect use of its certification marks.
C.6.5 The CB shall have procedures ensuring that the holders of certificates do not allow its certification mark be used in any way likely to lead to confusion among consumers.
C.7 Obligations of the CB relative to certifications
C.7.1 The CB shall not issue a certificate for any multi-ingredient organic product unless it verifies that the organic ingredients used in the product formulation have been certified as organic in accordance with part 13 of the SFCR by a CFIA accredited CB or to the terms of an organic equivalence arrangement by a CB accredited under the existing organic equivalency arrangements.
C.7.2 When a subjective judgment is required to determine compliance, the CB shall document explanatory information, assuring consistent and uniform application of the requirements and certification decisions.
C.7.3 The CB shall ensure that when it identifies and assigns responsibilities and tasks to members of its staff, impartiality is not in jeopardy.
C.7.4 The CB shall identify the management (committee, group or person) which will have overall responsibility for undertaking monitoring, inspection and certification activities as defined within the accreditation criteria, including execution of inspection, controls, evaluation and certification.
C.7.5 The CB shall have a signed legally enforceable agreement with each certificate holder that specifies the rights and responsibilities relevant to its certification activities including information about the CB appeal process and provisions to cover liabilities in situations where there is a significant risk of being sued.
C.7.6 The CB shall define and document the competence of the personnel for each function in the certification process including the VOs. The CB shall ensure that its personnel has professional training and experience relevant to the COR, including specific training with respect to the Canadian organic standards and the certification requirements outlined in C.2.
C.7.7 The CB shall have a signed agreement with the VO to refuse any work that would create a conflict-of-interest situation with the enterprise that is applying for certification, either because of a family link, or because of a business relationship with the applicant during the 12 months preceding its application to the CB.
C.7.8 The CB shall assign personnel to perform each evaluation task as per ISO 17065.
C.7.9 The CB shall establish procedures for evaluating and monitoring the performance of the personnel including the VO which should at minimum include initial assessment of competence and annual performance review, and regular field evaluation of the VOs. The frequency of the field evaluation shall be defined and developed by the CB. The CB may consider the number of VO inspections, VO's experience, and quality of the inspection reports in developing the frequency requirements. The CB shall record the performance of the personnel including the VOs' monitoring.
C.7.10 The CB shall document the estimated duration of on-site inspection, taking into account the intensity of the production system, the production type, the company's size, the results of the previous verification, complaints received and parallel production.
C.7.11 The CB shall document the minimum requirements for any audit trail, in relations to traceability.
C.7.12 The CB shall document its sampling and testing requirements.
C.7.13 The CB shall document its deadlines for presentation of the VO report to the CB.
C.7.14 The CB shall have procedures to address cases when an operator does not renew a certification from a previous year to ensure that the CB shall formally notify this operator in a timely manner that its certification is cancelled.
C.7.15 The CB shall exchange any information deemed confidential with other CFIA accredited CBs and/or CFIA to verify the validity of information on a holder of certificate. Such exchange is still considered to be and shall be managed as confidential by the receiving party.
C.8 Records control by the CB and operator
C.8.1 The CB shall document procedures to ensure it maintains a record system that complies with the SFCR requirements.
C.8.2 The CB shall ensure that its records are to be kept for a minimum of 5 years. This requirement shall also be documented by the CB.
C.8.3 The CB shall ensure that the operator maintains records and relevant supporting documents concerning the inputs, production, preparation and handling of crops, livestock and organic products that are or are intended to be sold, labelled or otherwise represented as organic in accordance with the CAN/CGSB-32.310 or CAN/CGSB-32.312 for a minimum of 5 years.
C.8.4 The operator shall submit this information to the CB, the CVB or the CFIA upon request.
C.9 CB records
C.9.1 The CB shall maintain all data listed below and shall provide it to the CVB and the CFIA annually by the end of the calendar year for each operator granted certification. For those elements of this information provided via the internet, it is acceptable to provide the URL to this information instead. For those elements of this information not provided via the internet, that information shall still be provided annually by the CB and the CVB shall transfer it to the CFIA.
- Legal (corporate) name of operator
- Full address of the operator's head office including phone numbers and fax numbers
- Type of operation (primary, processing or exporter)
- Generic names of the products certified
C.9.2 The CB shall maintain records of all major changes that took place during the previous year and that have affected corporate structure and directors, the administrative structure, the main managers of the organization and members of the committees. It shall provide this information to the CVB or the CFIA upon request.
C.9.3 The CB shall maintain records of all modifications made to policies, internal procedures and regulations governing the organization and its certification system. It shall provide this information to the CVB and/or the CFIA upon request.
C.9.4 The CB shall maintain records of the following:
- certificates newly issued, renewed, and withdrawn, listed by operator category under the COR
- number of annual inspections, number of annual inspections by activity, number of unannounced inspections and number of unannounced inspections by activity
- number of nonconformities issued
- number of samples collected
- number of complaints
- number of attestations of compliance issued
C.9.5 The CB shall submit this information to the CVB or the CFIA upon request.
C.10 Requirements when an operator changes a CB under COR
C.10.1 Requirements on the operator
C.10.1.1 The operator who decides to change their current CB (sending) to a new CB (receiving) shall submit an application for certification as a new applicant, complete an application form prescribed by the new CB (receiving) and follow the application requirements as per C.2.1 of the COR Operating Manual.
C.10.1.2 The operator, including those who intend to become part of a grower group, shall notify their current CB of their intent to change the CB and shall request a "letter of good standing" (appendix G) to be sent to the new CB (receiving), confirming that all nonconformities (NCs) and any contract conditions (for example, outstanding fees) have been addressed. The current CB shall send this letter directly to the new CB. If necessary, the receiving CB can directly request additional information to the sending CB.
C.10.1.3 The operator shall maintain their current certification until the new certification process is complete and the new CB has issued documents confirming the certification of the operator's products as per subsection 345(2) of the SFCR.
C.10.1.4 The operator shall stop using their certificate issued from the sending CB after the new certification process is complete and the operator has received the new certificate.
C.10.1.5 The operator shall not use up existing supplies of labels which identify their previous CB on products they produce from the moment the operator receives the new certificate. New labels identifying the new CB must be used at once.
C 10.1.6 The operator may sell certified prepackaged products labelled with the name of the previous CB as long as these products were packaged before the CB change and an inventory list was provided to both CBs.
C.10.2 Requirements on the sending (current) CB
C.10.2.1 The sending (current) CB shall, upon request by the operator, send a letter of good standing to the new CB (receiving) confirming that all NCs and any contract conditions (for example, outstanding fees) have been addressed by the operator. A letter of good standing shall only be issued when an operator is in the process of changing CB and, when all NCs have been addressed by the operator.
C.10.2.2 The sending CB shall continue to monitor the operator's compliance with COR requirements and shall ensure that the operator resolves any outstanding NCs before the new certificate is issued by the new (receiving) CB.
C.10.2.3 The sending CB shall notify the operator that it terminates the certification agreement with the operator and will no longer monitor the compliance of this operator once the new CB confirms that a new certificate has been issued to the operator.
C.10.2.4 Upon receiving confirmation from the new CB that a new certificate has been issued to the operator, the sending CB shall require the operator to immediately stop the use of any labels or advertising which identify the sending CB on the operator's products.
C 10.2.5 The sending CB shall allow the operator to retain a copy of the current certificate only when the operator can demonstrate that previously certified products are still in inventory.
C 10.2.6 The sending CB shall report the cancellation in a monthly report to the CFIA as a "cancellation due to a CB change".
C.10.3 Requirements on the receiving (new) CB
C.10.3.1 The receiving CB shall require the operator to submit an application for certification as a new applicant, complete an application form prescribed by the new CB (receiving) and follow the application requirements as per C.2.1 of the COR Operating Manual.
C.10.3.2 The receiving CB shall request information on the name of the applicant's current (sending) CB.
C.10.3.3 The receiving CB shall review the information provided by the sending CB including the letter of good standing.
C.10.3.4 The receiving CB shall schedule and conduct an on-site inspection of the operator's facility as per C.2.3 of the COR operating manual prior to making a certification decision.
C.10.3.5 The receiving CB shall issue a new certificate only after the certification process is complete and the applicant has been determined to be in compliance with all the COR requirements. The initial date on the new certificate shall be the date on which the receiving CB issued the certificate.
C.10.3.6 The receiving CB shall inform the sending CB within 5 working days that the receiving CB has issued a new certificate to the operator.
C.11 Requirements when a CB issues attestation of compliance
C.11.1 Scope
As per part 13 of the SFCR, a CFIA accredited CB upon request shall issue a document referred to as an "attestation of compliance" to a person that conducts physical activities with respect to the organic product (for example, slaughtering where the meat is not packaged and labelled, storing, seed cleaning and other custom services for bulk organic products where the ownership of the products remains with the primary producer/processor) which is not yet in an impermeable package, with the exception of retail and transport.
With respect to transportation of organic products which are not packaged or labelled, the CBs must verify that the organic integrity of the product is maintained by reviewing an affidavit signed by the truck company or other methods.
In cases when a service provider does not hold an attestation of compliance, it is the responsibility of the certificate holder to ensure that these services/activities are conducted in accordance with the COR requirements. These activities have to be included in the certificate holder/applicant's organic system plan and be verified by the CB as part of the certificate holder/applicant's on-site inspection.
C.11.2 Procedure for issuing attestation of compliance under COR
C.11.2.1 The CB shall verify that the activities are being conducted in accordance with CAN/CGSB 32.310 or CAN/CGSB 32.312 to maintain the integrity of the organic product.
C.11.2.2 The CB shall follow the steps outlined under C.2 of the COR operating manual as applicable to the activity conducted. As a minimum the CB shall:
- C.11.2.2.1 require the service provider to complete an application form
- C.11.2.2.2 request an organic plan and relevant documents which demonstrate how the integrity of the organic product is maintained
- C.11.2.2.3 verify compliance to CAN/CGSB 32.310 and CAN/CGSB 32.312 as applicable to the activity conducted
- C.11.2.2.4 conduct an annual inspection at a time when organic product is being handled or according to C.2.2.11
- C.11.2.2.5 conduct noncompliance follow-up according to C.2.3
C.11.2.3 The CFIA accredited CBs shall:
- C.11.2.3.1 issue an "attestation of compliance" using the template included in the Appendix 1 of the COR Operating Manual, which will be valid for 12 months beginning on the day on which it is granted
- C.11.2.3.2 suspend or cancel an "attestation of compliance" as required according to C.2.8
C.11.2.4 The CFIA accredited CBs shall accept an "attestation of compliance" issued by any CFIA accredited CB as meeting the SFCR requirements for maintenance of integrity and shall not require any further verification.
C.12 Requirements for grower group certification under COR
C.12.1 Requirements for grower group organizations
C.12.1.1 The grower group shall only seek certification with a CB accredited by CFIA under the COR that is accredited to certify grower groups.
C 12.1.2 The CVB shall assess the ability of the CB to perform the group certification and recommend it for accreditation to the CFIA. A CB shall be accredited for grower group scope if they have policies and procedures to verify compliance of the group and the individual group members.
C.12.1.3 The grower group composed of production units, sites, or facilities, shall be recognized as a "person" according to part 13 of SFCR.
C.12.1.4 The grower group may be organized on itself, that is, as a co-operative, or as a structured group of producers affiliated to a processor.
C.12.1.5 All members of the grower group shall apply similar production systems and should be in geographical proximity to each other. Only small farmers can be members of the group covered by group certification. Large farms can also belong to the group but have to be inspected annually by the CB.
C.12.1.6 The grower group shall be established formally, based on written agreements with its members. It shall have a central management, established decision procedures and be a legal entity.
C.12.1.7 The grower group shall have in place an effective and documented internal control system (ICS).
C.12.1.8 The management of the grower group shall sign a legally enforceable agreement with the CB specifying the responsibilities of both parties. The management shall obtain signed obligations from all grower group members to comply with the Canada Organic Standards and to permit inspection by the CB, the CVB or the CFIA.
C.12.1.9 The practices of the grower group operation shall be uniform and reflect a consistent process or methodology, using the same inputs and processes.
C.12.1.10 Participation in the grower group shall be limited to those members who market their organic production only through the grower group. A member of a grower group shall register to only one grower group for a given product. The maximum size of a grower group shall be 2,000 members.
C.12.2 Requirements for internal control system (ICS)
C.12.2.1 The grower group shall document and implement an internal control system (ICS) for supervision and documentation of production practices and inputs used at each sub-unit, and collected at each production unit, site, or facility. An identified person or body is responsible for verifying compliance with the Canada Organic Regime of each member of the group.
C.12.2.2 The internal control system shall include a contractual arrangement with each member of the grower group.
C.12.2.3 The internal control system shall be implemented by competent personnel including ICS manager and ICS inspectors. ICS inspectors designated by the grower group shall carry out internal controls.
C.12.2.4. Adequate number of ICS inspectors shall be identified from within the group based on the type, structure, size, products, and the activities of the group. The ICS inspectors shall be trained annually and their knowledge shall be assessed and documented at the end of the training.
C.12.2.5 The ICS inspectors shall carry out at least 1 annual on-site inspection visit to each individual member including visits to fields and facilities. Any additional risk-based inspections shall be conducted in accordance with the schedule and the procedures provided by the ICS manager.
C.12.2.6 The ICS inspectors shall draft internal inspection reports and submit it within a timeframe specified in the ICS to the ICS manager.
C.12.2.7 The internal control system shall contain appropriate records including:
- production description, production and/or preparation specifications for products to which the application applies
- maps, description of the farms and the facilities of all members
- list of inputs (ingredients and agricultural substances)
- a copy of organic production and/or preparation plans
- traceability records, including information on the quantities, on the following activities, where relevant:
- purchase and distribution of farm inputs including plant reproductive material by the group;
- production including harvest;
- storing;
- preparation;
- delivery of products from each member to the joint marketing system;
- placing on the market of products by the grower group.
- corrective actions required by the CB during the previous certification cycle, as well as any corrective measures implemented by members concerning these requests
- a complete list of registered group members
- continuous verification of implementation of the internal inspections
- summary of the internal inspection reports including the date of the last internal inspection with the name of the ICS inspector
- the training of members of the group on the ICS procedures and the requirements of COR
- the approval of new members in an existing group or, where appropriate, the approval of new production units or new activities of existing members upon the approval by the ICS manager on the basis of the internal inspection report
C.12.2.8 The internal control system shall have a mechanism to remove noncompliant group members from the list. The CB should be notified when a (noncompliant) member is sanctioned and/or when voluntarily withdrawn.
C.12.2.9 The internal control system shall record all nonconformities. The ICS shall require the member to respond to the NC report issued by the ICS within 30 working days of its receipt. The response shall either provide evidence of completion of corrective action taken to address each NC or present a plan with milestones as to how each NC will be addressed. This plan shall include a completion date not exceeding 90 working days from receipt of the NCs. The ICS shall accept times greater than those stated for the closure of a NC as long as they are justified and documented.
C.12.3 Initial certification
C.12.3.1 The CB shall evaluate the effectiveness of the ICS to assess compliance of all members with the requirements set out in CAN/CGSB 32.310, CAN/CGSB 32.311 and CAN/CGSB 32.312.
C.12.3.2 The certification inspection of the grower group by the CB shall include an assessment of the risks to organic integrity within the grower group and the geographical location in which it functions. A sample of all sites under the grower group's responsibility shall be subject to inspection visits by the CB to assess the effectiveness of the ICS. The CB may justify exceptions to this rule based on risk assessment.
C.12.3.3 The number of group members subject to the initial certification inspection shall be based on the results of a risk assessment and the following calculations.
C.12.3.3.1 Factors to define the risk as normal, medium and high shall include:
C.12.3.3.1.1 factors related to the magnitude of the grower group
- organisation size and sites' size
- value of the products
- numbers of years the grower group has functioned
- number of new members registered yearly
- staff turnover
- the management structure of the internal control system
- volume and value of the production
- the type of non-compliances
C.12.3.3.1.2 factors related to the characteristics of the grower group
- variations in the product systems
- risks for intermingling and/or contamination
- geographical dispersion of the sites
- degree of uniformity among the production units, sites or facilities
C.12.3.3.2 For normal risk situation, the number of group members subject to the initial certification inspection shall not be lower than the square root of the total number of units under the responsibility of the group.
C.12.3.3.3 If the risk is medium, the resulting number from C.12.3.3.2 shall be multiplied by 1.2.
C.12.3.3.4 If the risk is high, the resulting number from C.12.3.3.2 shall be multiplied by 1.4.
C.12.3.3.5 All calculation totals from C.12.3.3.2 – C.12.3.3.4 ending with decimals are to be rounded up.
C.12.3.4 The CB shall assign VOs who have appropriate training on inspection of internal control systems.
C.12.3.5 During the certification inspection the VO shall determine whether:
- C.12.3.5.1 all internal control documentation is in place
- C.12.3.5.2 internal inspections of all group members have been carried out annually
- C.12.3.5.3 new group members are only included after successful resolution of any NCs found during the internal inspection, according to the procedures agreed with the CB
- C.12.3.5.4 all noncompliances have been dealt with appropriately by the ICS
- C.12.3.5.5 inspection records have been maintained up to date by the ICS
C.12.3.6 The VO shall carry out a witness audit to determine whether the inspections of the ICS are conducted as written by ICS inspectors.
C.12.4 Maintenance of certification
C.12.4.1 Each year the CB shall define and justify a risk-based sample of members subject to annual inspection to assess the effectiveness of the ICS. The minimum number of members subject to annual CB inspection shall be square root of the total number of members multiplied by 1.5.
C.12.4.2 In cases of high risk members the CB shall increase the number of group members subject to annual inspection to at least 2 times the square root of the number of the members in the grower group (for example, ICS has issued a lot of internal sanctions, or a lot of new members).
C.12.4.3 The members visited by the CB shall be predominantly different from 1 year to another. In addition to the risk factors defined at C.12.3.3.1, the CBs may consider the following selection criteria when selecting the sites being subject to visits:
- results from internal control system inspection
- complaint files
- significant variations of the sites' size
- modifications since the last certification
C.12.4.4 The CB shall ensure that the grower group maintains an updated list of all members and informs the CB in a timely manner anytime there are changes to the status of the members and changes to the group as a whole.
C.12.4.5 The CB shall ensure that the grower group has established procedures for adding new members to the grower group.
C.12.5 Records
C.12.5.1 The CB shall ensure that the grower group has record-keeping protocols for the individual production units, sites, or facilities within a grower group.
C.12.5.2 The CB shall maintain records of sample inspection to ensure that over time, the inspections are representative of the grower group as a whole and take into account any previously identified risk.
C.12.6 Certification documents
C.12.6.1 The CB shall provide certification documents to the grower group as a whole. Members within a grower group that have had its operations or product certified cannot possess individual certificates unless that member has obtained its own certification independent from the grower group for a different product.
C.12.7 Suspension and cancellation
C.12.7.1 The CB shall hold the grower group as a whole responsible for compliance of all members.
C.12.7.2 The CB shall have a documented suspension policy in the event of noncompliance by the grower group or a member.
C.12.7.3 The CB shall suspend or cancel the certification granted to the grower group as a whole, in accordance with part 13 of the SFCR, in cases where the grower group's internal control system fails to act on these noncompliances.
Appendix A - Certificate template - Informative
Organic Certificate
Pursuant to part 13 of the Safe Food for Canadians Regulations (SFCR)
Issued by: ![]() (Insert CB name, address, contact information)
(Insert CB name, address, contact information)
Certification number: ![]()
Certificate Holder name and address: ![]()
This certification is based on compliance with CAN/CGSB 32.310 / CAN/CGSB 32.311 General Principles and Management Standards and Permitted Substances Lists
and/or
CAN/CGSB 32.312 Organic Production Systems -Aquaculture - General principles, management standards and permitted substances lists
and (if/when applicable)
Certified in accordance with the terms of the U.S. Canada Organic Equivalence Arrangement
Certification type and % organic content: ![]()
(Product addendum listing all certified products, including trademarks)
(Location of all production units covered by this certification (address, town, province/state, country) (includes but is not limited to: all production, processing, harvest and storage sites) (not applicable to grower group members)
Date on which the certification was initially granted: ![]()
Date of last inspection: ![]()
Date by which the operator shall submit application for subsequent annual inspection: ![]()
This certification remains valid unless suspended or cancelled by the ![]() (Insert the name of the CB) pursuant to part 13 of the Safe Food for Canadians Regulations (SFCR)
(Insert the name of the CB) pursuant to part 13 of the Safe Food for Canadians Regulations (SFCR)
Signed by (name and signature):
(CB authorized representative)
Dated:
Appendix B – Attestation of compliance template - Informative
Attestation of Compliance
Pursuant to part 13 of the Safe Food for Canadians Regulations (SFCR)
Issued by: [name of CB]
[Address of CB]
Verified Enterprise: [enterprise name]
[Enterprise address – line 1]
[Address – line 2]
Enterprise number: [number]
Type of Service: [type of service]
Effective period of verification: [12-month period]
This attestation is based on the requirements of the Canadian Organic Production Systems General Principles and Management Standards CAN/CGSB-32.310 and Permitted Substances Lists CAN/CGSB-32.311, as amended from time to time.
This document expires at the end of 12 months (the termination date of the effective period identified above) or when cancelled by the [CB name].
This document confirms that the products listed on page 2 are handled in compliance with the CAN/CGSB-32.310 and CAN/CGSB-32.311 and part 13 of the Safe Food for Canadians Regulations (SFCR).
CB's representative signature:
Print name:
Date of issue:
Appendix C - The family of certification documents
Documentation requirements for verification of continued organic integrity under the Canada Organic Regime
(Interpretation of part 13 of the SFCR)
The "family" of certification documents
Because an organic product carries its certification until the next point of transformation, operators may require different types of document to attest to a product's organic status and integrity. These documents are:
- organic product certificate
- certificate of packaging and labelling activities
- attestation of compliance (for a service provider conducting activities, excluding packaging and labelling activities)
These documents are considered the "family" of documents, which can be issued by a CFIA-accredited certification body (CB) in order to attest to the organic integrity of a product. For products and ingredients imported to Canada, parties within the Canada Organic Regime (COR) shall recognize organic certificates from equivalent organic systems.
The COR operating manual requires that all CFIA-accredited CBs accept certification documents including attestation of compliance, issued by another CFIA-accredited CB or any CB recognized under equivalency arrangements as meeting the SFCR requirements for the maintenance of organic integrity.
Whenever a document is issued, there must be verification that the operator requesting certification complies with the Canadian Organic Standards, such as requirements related to the organic plan.
An operation must hold more than one certification document depending on the specific activities in which they are involved in. For example, a product certificate cannot cover activities done on products that are not under the ownership of the person doing the activity. Depending on the scenario, a Packaging and labelling certificate and/or an Attestation of compliance must be issued.
| Type of documentation | Description |
|---|---|
| Organic product certificate | CBs shall issue certificates confirming the organic status of a product, verified to be produced or processed in compliance with the Canadian Organic Standards as per subsection 345(2) of the SFCR. Product certificates do not expire; however, subsection 346(1) of the SFCR requires the certification holder to submit updated information annually. |
| Certificate of packaging and labelling activities | CBs shall issue certificate of packaging or labelling activities to an operator as per subsection 348(2) of the SFCR. |
| Attestation of compliance | CBs may issue an attestation of compliance confirming that the service provider conducted an activity on behalf of an organic operator in compliance with the Canadian Organic Standards as per paragraph 344(2)(c) of the SFCR. The CB shall issue the attestation of compliance in accordance with C.11 of the COR operating manual. |
| Type of document | Expiry/Renewal |
|---|---|
| Organic product certificate | Does not expire. A certificate must be issued for an organic product on an annual basis as per subsection 345(2) of the SFCR. |
| Certificate of packaging and labelling activities | Remains in effect for a period of 12 months beginning on the day on which it is granted as per subsection 348 (3) of the SFCR. |
| Attestation of compliance | Remains in effect for a period of 12 months beginning on the day it was granted as per C.11 of the COR operating manual. |
| # | Operator | Scenario | Type of document |
|---|---|---|---|
| 1 | Farmer (primary producer) | The primary producer sells an organic product to a retailer or manufacturer without any processing or transformation. | Organic product certificate Any transport or handling of the product included in the organic plan by the farmer is covered by this certificate. Subsequent activities such as processing, transformation, packaging, and labelling by other parties are not covered by the organic product certificate issued to the primary producer. |
| 2 | Trader - Domestic distributor | The trader sells an organic product to a retailer such as bulk products obtained from a farmer or manufacturer. The product has not been transformed but the trader wishes to show that the organic integrity of the product has been maintained. | Organic product certificate The trader may apply for product certification and sell the organic product under this documentation (for example as the organic integrity has been verified, the name and address of the supplier are not required to be disclosed to the buyer). However, traders or distributors can still trade organic products without applying for organic product certificate, provided that organic integrity has not been compromised and the full documentation chain for these products is on hand and provided as required by either the certification body or the CFIA inspectors. |
| 3 | Trader - Exporter – Equivalency arrangement "Certificate of Inspection" / "Transaction Certificate" / "Export Certificate" required | The exporter sells an organic product to an export market with whom Canada has an equivalency arrangement and the competent authority of the export market requires a "Certificate of Inspection" as is the case with the European Union, Switzerland, Japan and Taiwan. | Organic product certificate and "Certificate of Inspection" / "Transaction Certificate" / "Export Certificate" The applicable certificate should be issued by the CB that certified the product as organic. |
| 4 | Trader - Exporter – Equivalency Arrangement – "Certificate of Inspection" not required | The exporter sells an organic product to an export market with whom Canada has an Equivalency Arrangement but no "Certificate of Inspection" is required (for example United States and Costa Rica) | Organic product certificate Canadian organic products exported to the U.S. under the USCOEA must be accompanied by a valid organic product certificate issued by a Canadian Food Inspection Agency (CFIA) accredited certification body, which includes the following attestation statement, "Certified in compliance with the terms of the U.S.-Canada Organic Equivalency Arrangement". |
| 5 | Trader - Exporter – No Equivalency Arrangement | The exporter sells an organic product to an export market with whom Canada does not have an Equivalency Arrangement | Organic product certificate should be issued if the product is marketed in Canada. Import requirements of the country where the product will be marketed must be met. |
| 6 | Re-packager/Re-labeller (processor) | An operator purchases an organic product but changes its container (for example from bulk to single-package), changes its packaging, or provides a new label (for example changing or adding information to the original package). | Organic product certificate |
| 7 | Retailer (sale of bulk product from bins) | A retailer purchases bulk product for sale in its store and back-fills the bins as levels get lower. These bins are labelled "organic" and carry the Canada Organic Logo. | Organic product certificate Retailers, who choose to blend, further prepare, package or label organic products and use the Canada Organic Logo are required to obtain certification under the federal system. |
| 8 | Retailer (repackaging of bulk product into small packages) | A retailer purchases bulk product for sale in its store and re-packages (for example makes individual-sale units available). These packages are labelled "organic" and carry the Canada Organic Logo. | Organic product certificate Retailers, who choose to blend, further prepare, package or label organic products but choose not to use the Canada Organic Logo, and do not cross provincial lines, are not required to obtain certification under the federal system. The Canada Organic Retailing Practices Guide is recommended for best management practices in such situations. However, in certain provinces, the retailer may have to obtain certification under a provincial regime |
| 9 | Brand owner (products under a private label) | A brand owner buys (under contract) prepackaged products via third-party producers or manufacturers and markets them under their own brand of product. | Organic product certificate Brand owner may apply for certification of the product, however, it is not mandatory. When the brand owner is a certificate holder, it is not necessary to disclose the name and address of the original supplier on the packaging as long as the brand owner's name and address is included. When the brand owner is not a certificate holder, for consumer access to information about the product, consideration could be given to providing the name and address of the original supplier on the final packaging. The label must display the name of the CB which issued the final organic product certificate. |
| 10 | Manufacturer/ processor | A manufacturer/ processor buys organic product from a trader or producer and transforms it into a new product. | Organic product certificate All organic ingredients must be accompanied by their organic product certificate, and the manufactured/processed products must be issued organic product certificates by a CB under the COR. |
| 11 | Contract packaging and labelling activities | Contract service providers that package and label organic products on behalf of the organic product certificate holder (as per SFCR paragraph 344 (2) (d)) | Certificate of packaging and labelling activities |
| 12 | Contract services excluding packaging and labelling and final product preparation for example services conducted on behalf of the organic product certificate holder. | Contract service providers excluding packaging and labelling (for example for slaughter, transport, storage, seed cleaning, etc.) who perform contractual work for operators in relation to certified organic products but the ownership of the product continuously rests with the holder of the organic certificate. Note: complex operations depending on individual circumstances are required to hold more than one type of document (for example, a butcher who provides the services of meat cutting AND packaging and labelling of products on behalf of an organic product certificate holder). | Attestation of compliance
|
| 13 | On-site services or equipment (for example mobile juicers) | A certified operation (for example a farm) leases or loans equipment or has services that are performed on-site, but the ownership of the organic product continuously rests with the organic product certificate holder. | None If the organic plan includes this situation, and the CB is able to verify compliance to the standards, including cleaning requirements, then the equipment or service may be covered by the original organic product certificate. or Attestation of compliance May be issued in accordance with section C.11 of the COR Operating Manual. |
Appendix D - CB management of nonconformities and enforcement actions under the Canada Organic Regime
Click on image for larger view 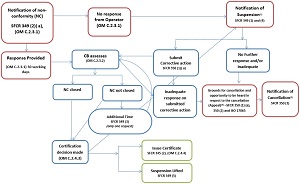
Description for flow chart showing the CBs regulatory authorities for enforcement actions under Part 13 of the SFCR.
- The CB issues a notification of nonconformity (NC) to an operator/applicant and requests that the operator responds to the notification within 30 working days of its receipt, as per paragraph 349(2)(a) of the SFCR and section C.2.3.1 of the COR operating manual. The response shall either provide evidence of the completion of corrective action(s) taken to address the nonconformity or, a plan with milestones as to how the nonconformity will be addressed.
- After the response is provided, the CB assesses the information and verifies that the operator has addressed all the nonconformities before determining whether to close the NC. If the NC is closed, the CB makes a certification decision. Depending on the circumstance, the certification decision could be either to lift a suspension of an existing organic operator or to issue organic certification to an applicant/operator.
- If the CB determines that the NC is not closed, the operator may request for a 1 time extension from their CB in order to submit their corrective action plan to address the nonconformity. The CB then reassesses the information provided and determines whether to close the NC. If the NC is closed a certification decision will be made. If the NC is not closed, the CB issues a notification of suspension to the operator as per subsections 349(1) and 349(4) of the SFCR.
- The CB issues a notification of nonconformity (NC) to an operator and requests that the operator responds to the notification within 30 working days of its receipt, but the operator does not provide a response. In this case, the CB must issue a notice of suspension to the operator in accordance with subsections 349(1) and 349(4) of the SFCR.
- After the CB issues a notification of suspension to an operator, the operator has 30 days to take corrective action or submit a corrective action plan to their CB as per paragraph 350(1)(a) of the SFCR. The CB assesses the information provided and determines whether or not the information is adequate.
- If the information is adequate, the CB determines whether or not to close the NC and a certification decision is made accordingly.
- If the information is inadequate, the CB notifies the operator of the grounds for cancellation and provides the operator an opportunity to be heard.
- The CB issues the operator a notification of cancellation as per section 350 of the SFCR.
- After the CB issues a notification of suspension to an operator, if the operator did not provide a response or provided the CB with an inadequate response, the CB must notify the operator of the grounds for cancellation and provide the operator an opportunity to be heard.
- The CB issues the operator a notification of cancellation as per section 350 of the SFCR.
Additional information
- The Notification of Suspension has to be issued separately from the Notification of Proposed Cancellation (that is, grounds for cancellation).
- Suspension and cancellation are 2 separate processes. Only in the case of paragraph 350(1)(b) of the SFCR that the CB can proceed directly with a Notification of Proposed Cancellation without suspending the operator. The CB has to give the operator the "opportunity to be heard".
- Under the SFCR the CBs are required to have an appeal process in line with ISO/IEC17065 and the operator has to be informed about this process when notified about cancellation. This process is considered compliant with the SFCR requirement for "opportunity to be heard".
Appendix E - CVB Management of nonconformities and enforcement actions under the Canada Organic Regime (Initial Application/Reassessment for Accreditation (First part))
Click on image for larger view 
Description for flow chart showing CVB management of nonconformities and enforcement actions under the Canada Organic Regime Appendix E: Initial Application / Reassessment for Accreditation (1st part):
- The Conformity Verification Body (CVB) conducts an on-site visit and issues a report to the applicant Certification Body (CB) as per section B.2.2.17 of the Canada Organic Regime (COR) operating manual. The applicant CB has 30 working days from report receipt to submit responses to resolve any NCs.
- If the response is submitted on time and the actions taken/responses are sufficient and effective to resolve NCs, CFIA accepts CVB recommendation and grants accreditation to the applicant CB.
- In order for the CVB to properly assess if actions taken/responses are sufficient and effective to resolve NCs, further info may be requested and/or assessment activities may be conducted. Also, In the case of reassessment, the CVB needs to consider the expiration date of CB current accreditation letter and manage any additional period granted.
- If the response is submitted in a timely manner and the actions taken/responses are sufficient and effective to resolve NCs: CFIA accepts CVB recommendation and grants accreditation.
- In the case where the responses are not submitted on time and/or found to be unacceptable, CFIA accepts the CVB recommendation and does not grant accreditation to the applicant CB. CVB sends notice of refusal in writing to the applicant CB with rationale and obtains confirmation of receipt. CB has the right to request that CFIA reviews the CVB decision within 30 days from notice receipt as per SFCR part 13 section 362.
- In the case of reassessments, follow Appendix F from point 4 onwards (in the description).
- Applicant CB's request for review is sent to CFIA:
- CFIA reviews CVB decision.
- If CFIA does not agree with the CVB decision and accepts the applicant CB's application, CFIA accredits the CB and notifies the CB and CVB in writing as per SFCR part 13 subsections 361(1) and (2) and section 363.
- If Applicant CB does not request a review:
- CFIA confirms the CVB decision. CFIA notifies the applicant CB in writing that accreditation was refused as per SFCR part 13 section 363.
Appendix F - CVB Management of nonconformities and enforcement actions under the Canada Organic Regime (Surveillance and Monitoring / Reassessment for Accreditation (Second part))
Click on image for larger view 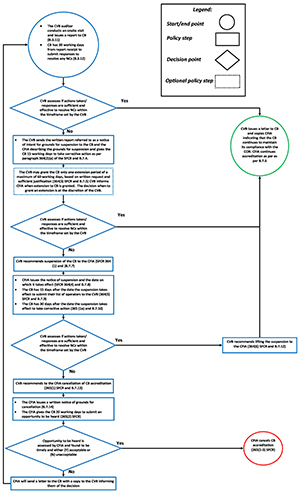
Description for flow chart showing CVB management of nonconformities and enforcement actions under the Canada Organic Regime Appendix F: Surveillance and monitoring / Reassessment for Accreditation (2nd part):
- The Conformity Verification Body (CVB) conducts an onsite visit and issues a report to the Certification Body (CB) as per section B.3.11 of the Canada Organic Regime (COR) operating manual. The CB has 30 working days from report receipt to submit responses to resolve any NCs.
- If the response is submitted on time and is accepted by the CVB, the CVB issues a letter for continuation of CB accreditation with a copy to CFIA and CFIA continues CB accreditation as per B.7.6.
- In the case where the responses are not submitted on time and/or found to be unacceptable, the CVB sends the written report referred to as a notice of intent for grounds for suspension to the CB and the CFIA describing the grounds for suspension and gives the CB 15 working days to take corrective action as per paragraph 364(2)(a) of the SFCR and B.7.4.
- The CVB may grant only 1 extension period upon written request from the CB supported by justification as per subsection 364(3) of the SFCR. The length of the extension period is at the discretion of the CVB but should not be longer than 60 working days. CVB informs CFIA when extension to CB is granted. The decision when to grant an extension is at the discretion of the CVB.
- In the case when the CVB is satisfied that the CB has resolved the issues that were grounds for suspension within the time allowed, the CVB notifies the CFIA in writing to recommend continuation of accreditation.
- In the case where the CVB is not satisfied with the CB's corrective actions within the time allowed, the CVB recommends suspension to the CFIA as per subsection 364(1) of the SFCR.
- The CFIA issues the notice of suspension and the date on which it takes effect as per subsection 364(4) of the SFCR.
- The CB has 15 days after the date the suspension takes effect to submit their list of operators as per subsection 364(5) of the SFCR.
- The CB has 30 days after the date the suspension takes effect to take corrective action as per subsection 365(1a) of the SFCR.
- The CFIA issues the notice of suspension and the date on which it takes effect as per subsection 364(4) of the SFCR.
- In the case when the CVB is satisfied with the CB's corrective actions within the time allowed, the CVB recommends lifting the suspension to the CFIA as per subsection 364(6) of the SFCR.
- The CVB issues a letter for continuation of CB accreditation and CFIA continues CB accreditation as per SFCR subsections 361(1) and (2).
- In the case when the CVB is not satisfied with the CB's corrective actions within the time allowed, the CVB recommends to the CFIA cancellation of accreditation as per subsection 365(1) of the SFCR.
- The CFIA issues a notice in writing for grounds for cancellation to the CB and gives the CB opportunity to be heard as per subsection 365(2) of the SFCR. The CFIA allows 20 working days to the CB.
- In the case when the opportunity to be heard is made within the time allowed and is found acceptable by the CFIA, CFIA will send a letter to the CB with a copy to the CVB informing them of the decision.
- In the case when the opportunity to be heard is not made within the time allowed and/or is found unacceptable, the CFIA issues accreditation cancellation to the CB as per subsections 365(1-3).
Appendix G - Letter of good standing for an operator changing CB under the Canada Organic Regime (Template) - Informative
Letter of good standing for an operator changing CB under the Canada Organic Regime (Template)
Issued by: [insert CB name and address]
Issued to: [insert operator name and address]
Type of certification document: [for example organic product certificate, attestation of compliance]
Type of service (in the case of attestation of compliance): [for example seed cleaning]
Expiration date of certificate/attestation (specify for certificate of packaging and labelling activities and attestation of compliance):
Date of next application and fee due:
Date of next inspection:
This letter is issued to [Operator name] to confirm that there are no outstanding nonconformities including any contract conditions (for example fees) and that [Operator name] certification is in good standing.
CB's representative signature:
Name of CB representative:
Date letter issued:
- Date modified: