Chapter 3 - Testing
This page is part of the Guidance Document Repository (GDR).
Looking for related documents?
Search for related documents in the Guidance Document Repository
3.1 Tuberculosis testing (updated January 2017)
1. This section deals with tuberculosis testing procedure only. Information about the disease, bovine tuberculosis, may be found on the CFIA website.
2. Tuberculin skin testing of livestock has been the cornerstone of on-farm active surveillance for the National Bovine Tuberculosis (TB) Eradication Program (NBTEP) for many years. Testing is also applied for the purpose of import, export, entry into semen production centres, and to support disease investigations. The basis of tuberculin testing is the induction of delayed-type hypersensitivity reaction to the intradermal injection of antigenic substances derived from a laboratory strain of Mycobacterium bovis (M. bovis). The hypersensitivity response results in the accumulation of cells and fluid in the dermis which creates swelling at the injection site. In test positive animals, the intradermal skin reaction develops within 24 hours post-injection, reaching a maximum by 48-72 hours, characterized by erythema and swelling, but rarely to the point of induration or ulceration.
3. There are two limitations associated with intradermal tuberculin tests:
- Some animals infected with M. bovis fail to respond to the test (false negatives). The number of animals in this category is a measure of the sensitivity of the test; the probability of the test correctly identifying as reactors those animals that are truly infected. False negative responses may be observed for a variety of reasons, including compromised tuberculin quality, improper testing technique, the chronicity and extent of the disease process, or variations in the time of testing in relation to calving and previous tuberculin tests.
- Some animals that are not infected with M. bovis react to the test (false positives). The number of animals in this category is a measure of the specificity of the test; the probability of the test correctly identifying as negative those animals that are truly not infected. Most false positive responses are due to the animal having been exposed to antigens similar to, or shared with, those in tuberculin.
4. Sensitivity and specificity are characteristics of the tuberculin skin test itself and are not influenced by the population on which the test is applied (i.e. if the population is TB-free or actually has TB at a given prevalence). However, what is influenced by the prevalence of TB in a population is the probability that a reactor animal is truly infected with M. bovis (the positive predictive value of a positive test result). Positive and negative predictive values are influenced by the prevalence of disease in the population that is being tested. The higher the prevalence in a population, the more likely that an animal that reacts to the tuberculin test actually has the disease, than if the test were performed in a population with a low prevalence.
Tuberculin procurement, handling, storage, use, and disposal
5. All tuberculins used in Canada are produced by the Biologics Production Unit of the Canadian Food Inspection Agency (CFIA) Ottawa Laboratory Fallowfield (OLF). Bovine purified protein derivative (PPD) tuberculin, produced by the CFIA, contains 3250 IU in each 0.1 mL.
6. Tuberculin is distributed by district offices to accredited veterinarians. Accredited veterinarians that wish to obtain tuberculin are to contact their CFIA district office who will provide tuberculin upon request free of charge.
7. In light of the short turnaround time for tuberculin, it is unnecessary to stockpile an inventory in anticipation of future needs. Stockpiling leads to unnecessary and increased wastage of tuberculin and contributes to supply shortages.
8. Tuberculin has an expiry date of one year from the time of potency testing, when stored and handled appropriately, but, ideally, should be used within a few months of receipt. Manage tuberculin inventories carefully to avoid their expiry before use. A test performed with expired tuberculin is invalid.
9. Always check the expiry date of tuberculin before use. Do Not Use Expired Tuberculin. A tuberculin test is valid only if carried out prior to, or on the same date as, the expiration date on the bottle. If expired tuberculin is used inadvertently to conduct an official TB test, the test is invalid and will have to be repeated after the appropriate period of time has passed, as required for the particular tuberculin test procedure. Contact immediately the district office for further instructions.
10. To be reliable, tuberculin must be handled and used according to strict parameters:
- Store tuberculin in a cool environment (4°C), and protect from light. Tuberculin exposed to extreme temperatures or to sunlight for more than a few hours is improper for use.
- Always pack tuberculin on ice or frozen cold packs during transit.
- Use a good aseptic technique when handling tuberculin. To prevent contamination of tuberculin, disinfect the stopper of the vial after peeling back the aluminum seal.
- Immediately dispose of any expired tuberculin as follows:
- Discard directly as biological waste; or
- Return to the CFIA district office for disposal (only full and unopened bottles)
- Please also return any tuberculin which is no longer intended for field use (only full and unopened bottles).
11. Be aware that tuberculin is for use only by trained and qualified accredited veterinarians.
Tuberculin testing notes
12. Testing may involve considerable travel time and many hours to process all animals to be tested. To facilitate this process, it is important to prepare in advance to ensure that all materials will be available at the test site.
Syringes and needles
13. Use 1 ml tuberculin syringe. Only a single dose may be drawn at once. Multiple doses in the same syringe are not allowed.
14. Use a new needle for each animal: 26 gauge, ⅜" long.
15. Be aware that tuberculin syringes may be re-loaded, with a new needle for each animal.
16. Do not pre-load syringes with tuberculin prior to testing under any circumstances.
Test available
17. Tuberculin tests may only be performed by trained and qualified accredited veterinarians as follows:
- Caudal fold tuberculin (CFT) test for bovine TB testing in cattle, bison, goats or sheep.
- Mid-cervical tuberculin (MCT) test for bovine TB testing in cervids.
- Single post-axillary test for bovine TB testing in camelids
- Base-of-the-ear tuberculin test for bovine TB testing in swine
18. For accredited veterinarians, training and qualification entails having successfully completed:
- Review of this section of the Manual with a district veterinarian; and
- On farm demonstration and practice on live animals with a CFIA veterinarian. The district veterinarian must keep a record in the accredited veterinarian's file regarding the date and place where the injection technique was practised on farm.
19. For accuracy of test results conducted under the NBTEP, it is essential that the same person conduct both the injection-day and reading-day measurements for these tests, other than for exceptional circumstances that make this impossible (unplanned and unavoidable absence).
20. The base-of-the-ear tuberculin test for swine is not an official test for bovine TB under the NBTEP and is only performed under export programs testing for live swine and swine semen. Given the volume of animals that are sometimes tested, the requirements of biosecurity protocols to enter a swine premises and the bovine TB freedom of commercial swine operations in Canada, injection-day and reading-day inspections are to be conducted by the same trained person whenever possible and practical.
Adjunct activities
21. If the owner or operator plans to medicate the animals (e.g. vaccines, anti-parasitic) while the animals are being handled for TB testing, all medications shall be applied at the time of the tuberculin testing reading, not at the time of tuberculin injection. This facilitates the salvage of carcasses for human food, if animals must be destroyed due to test results or injury, and ensures that the medication does not interfere with the test results.
Test date
22. The date of the tuberculin test is to be recorded on the report of intradermal test, including both the tuberculin injection date and the reading date. For export purpose and entry into a semen production centre, the test date recorded is the reading date.
23. For the purpose of calculating the interval that has elapsed since a tuberculin test was performed, use the tuberculin injection date.
Caudal fold tuberculin (CFT) test
Preparation
24. The CFT test is the standard official screening test for bovine TB in cattle, bison, sheep and goats at low risk of having tuberculosis.
25. The test involves an intradermal injection of 0.1 mL of bovine PPD tuberculin into either caudal fold, with observation and palpation at 72 hours ± 6 hours.
26. Do not apply the CFT test to an animal that has had a tuberculin test of any kind during the preceding 60 days.
27. Prepare the following equipment:
tuberculin – bovine PPD tuberculin (store in dark at 4°C)
syringes – 1 mL tuberculin syringes
needles – 26 gauge, ⅜" long
absorbent cotton
Form CFIA/ACIA 1524 – Report of Intradermal Test
headlamp or flashlight, if poor lighting
28. Adequately restrain each animal for testing to ensure correct animal identification, proper preparation of the injection site, careful tuberculin injection, and reading of the test result. Do not apply or observe the test without having the animal adequately restrained.
29. Record on Form CFIA/ACIA 1524 the individual animal identification of each animal tested. Acceptable animal identification is as follows: a tag approved (or considered equivalent) under the Livestock Identification and Traceability (TRACE) Program, a Health of Animals (HofA) ear tag, or a registered tattoo.
30. The injection site is the caudal fold, distal to the base of the tail, well away from the hairline, in the center of the fold. Use either the left or right fold, but be consistent. If a lesion is encountered on the caudal fold use the opposite fold and make a note of this change.
Injection
31. Clean the caudal fold with cotton (moistened with water, if necessary). Do not use alcohol to clean the injection site. Note any abnormalities, lesions, or scabs observed in the vicinity of the injection site, recording on Form CFIA/ACIA 1524 to avoid being mistaken for tuberculin responses.
32. Check tuberculin label to confirm type – M. bovis tuberculin has an orange label.
33. Check/confirm the tuberculin expiry date against current date.
34. Disinfect rubber stopper with alcohol after opening aluminium seal.
35. Fill tuberculin syringe to 0.1 mL volume. Only a single dose can be drawn.
36. Raise tail and locate the caudal tail folds.
37. Insert needle into the dermis of the skin on the center line of either the left or right caudal tail fold, distal to the tail base and away from the hair line. Be consistent and use either the right or left caudal fold for all test animals on the farm. Lift the tip of the needle slightly after insertion to ensure it is clearly outlined under the skin. Inject 0.1 mL of tuberculin intradermally and withdraw the needle slowly.
38. View injection site to ensure that the injected tuberculin is visible and palpable as a definite bleb. If not, it indicates that either the needle was inserted too deeply or the dose was inadequate. The administration of tuberculin should be repeated on the opposite side.
39. Discard needle immediately into a sharps container.
Reading
40. After 72 hours ± 6 hours, restrain the animal and confirm its identification.
41. Raise the tail with one hand to slightly stretch the caudal tail folds.
42. Observe and palpate the length of the caudal fold injection site with the thumb and index finger of the other hand.
43. Record the response to the CFT test for each animal on Form CFIA/ACIA 1524) as follows:
- Negative (Neg.): There is no visible or palpable change in tissue at the site of injection; or
- Reactor: There is any visible or palpable disturbance in tissue at the site of injection. Contact the district office immediately.
When in doubt regarding the nature of the response to the CFT test, classify the animal as a reactor.
44. Animals classified as reactors on the CFT test are retested by CFIA veterinarians, using the Comparative Cervical Tuberculin (CCT) Test (refer to the CCT test section below).
45. All tuberculin tests are official and must be recorded and reported to a CFIA District office by one of the following methods:
- Fully completed Form CFIA/ACIA 1524, including animal information for each animal tested. A copy of the completed Form CFIA/ACIA 1524 is forwarded to the district office responsible of the area where the animals were tested. A copy must also accompany the export health certificate sent for endorsement to the district office (if different from the district where the animals were tested) or the certificate of health for entry into a semen production centre (Form CFIA/ACIA 1634 – Certificate of Health for Entry into a Semen Production Centre or Isolation worksheet and Authorization for Release); or
- Partially completed Form CFIA/ACIA 1524 as follows and attach an Animal List.
- Complete all parts of Form CFIA/ACIA 1524 (except the animal information section) including the signature block where the accredited veterinarian certified that he/she tested the animals described on date and at location shown.
- Attach to Form CFIA/ACIA 1524 a list of animals that were TB tested and found negative. In the animal information section of the Form CFIA/ACIA 1524, insert a statement that reads "The (insert # of animals) (insert species) listed on attached list were tested on the date shown above and at the location shown above with negative results". The accredited veterinarian should sign and date the list.
- Any animals with non-negative results must be included in the animal information section of Form CFIA/ACIA 1524.
- A copy of the completed Form CFIA/ACIA 1524 and the attached list are forwarded to the district office responsible of the area where the animals were tested. A copy must also accompany the export health certificate sent for endorsement to the district office (if different from the district where the animals were tested) or the certificate for entry into a semen production centre (Form CFIA/ACIA 1634 or Isolation worksheet and Authorization for Release).
Monitoring CFT testing: performance standard
46. The CFT test has less than perfect specificity (ranges from 97-99%), resulting in a false positive reactor rate in a disease-free livestock population of 1%–3% per year. False positive reactors are an indicator that the test is being performed correctly as they are expected when applying this test in a group of animals without disease.
47. A minimum performance standard exists for all inspectors and veterinarians, including accredited veterinarians who conduct CFT testing. The reactor rate of each accredited veterinarian trained to conduct CFT test shall be monitored and calculated based on the number of cattle or bison test for each one year period. If a reactor rate less than the minimum performance standard of 1% is identified, this will be addressed and appropriate actions taken (i.e. additional training or supervision, audit) and documented. If insufficient cattle/bison are tested in one year for evaluation of compliance with the standard, the period for review will be extended.
48. District veterinarians involved in the evaluation of official services performed by accredited veterinarians are responsible for monitoring reactor rates of accredited veterinarians. Failure to meet the minimum performance standard (i.e. non-conformance) noted during evaluation of accredited veterinarians services will be addressed according to the Procedures Manual – Management of Accredited Veterinary Services – to improve the services provided.
49. Authorization to perform CFT test may be withdrawn for accredited veterinarians where non-conformance to the minimum performance standard cannot be resolved.
Mid-cervical tuberculin (MCT) test
Preparation
50. The MCT test is the standard official screening test for tuberculosis in cervids. The test involves an intradermal injection of 0.1 mL of bovine PPD tuberculin in the mid-cervical area, with observation and palpation at 72 hours ± 6 hours.
51. Do not apply the MCT test to an animal that has had a tuberculin test of any kind during the preceding 60 days.
52. Prepare the following equipment:
tuberculin – bovine PPD tuberculin (store in dark at 4°C)
syringes – 1 mL tuberculin syringes
needles – 26 gauge, ⅜" long
absorbent cotton
electric clippers with #40 head
Form CFIA/ACIA 1524 – Report of Intradermal Test
headlamp or flashlight, if poor lighting
53. Adequately restrain each cervid for testing to ensure correct animal identification, proper preparation of the injection site, careful tuberculin injection, and proper reading of the test result. Do not apply or observe the test without having the animal adequately restrained.
54. Record on Form CFIA/ACIA 1524 the individual animal identification of each animal tested. Acceptable animal identification is as follows: tag approved (or considered equivalent) under the Livestock Identification and Traceability (TRACE) program, a HofA ear tag, a registered tattoo, or an ear tag approved by a provincial regulatory authority for farmed cervids.
Injection
55. Use clippers to clip an area in the mid-cervical region that is approximately 8-cm square.
56. Clean the injection site with cotton (moistened with water, if necessary). Do not use alcohol to clean the injection site. Note any abnormalities, lesions or scabs observed in the vicinity of the injection site, recording on Form CFIA/ACIA 1524 to avoid being mistaken for tuberculin responses.
57. Check tuberculin label for type – M. bovis tuberculin has an orange label.
58. Check/confirm expiry date against current date.
59. Disinfect rubber stopper with alcohol after opening aluminium seal.
60. Fill tuberculin syringe to 0.1 mL volume.
61. Insert needle into the dermis of the skin. Lift the tip of the needle slightly after insertion to ensure it is clearly outlined under the skin. Inject 0.1 mL of tuberculin intradermally and withdraw the needle slowly.
62. View injection site to ensure that the injected tuberculin is visible and palpable as a definite bleb. If not, it indicates that either the needle was inserted too deeply or the dose was inadequate. In such case, the administration of tuberculin should be repeated on the other side of the neck if possible, or at least 15 cm rostral or caudal from the previous injection site.
63. Discard needle immediately into a sharps container.
Reading
64. Observe and palpate the injection site at 72 hours ± 6 hours post-injection. Good lighting is essential.
65. Record the responses to the MCT test for each animal on Form CFIA/ACIA 1524 as follows:
- Negative (Neg.): There is no visible or palpable change in tissue at the site of injection; or
- Reactor: There is any visible or palpable disturbance in tissue at the site of injection. Contact the district office immediately.
When in doubt with respect to the nature of the response to the MCT test, classify the animal as a reactor.
66. Any animal that is classified as reactor on the MCT test is retested by a CFIA veterinarian with the Comparative Cervical Tuberculin (CCT) test (refer to the CCT test section below).
67. All tuberculin tests are official and must be recorded and reported to a CFIA District office by one of the following methods:
- Fully completed Form CFIA/ACIA 1524, including animal information for each animal tested. A copy of the completed Form CFIA/ACIA 1524 is forwarded to the district office responsible of the area where the animals were tested. A copy must also accompany the export health certificate sent for endorsement to the district office (if different from the district where the animal was tested); or
- Partially completed Form CFIA/ACIA 1524 as follows and attach an Animal List.
- Complete all parts of Form CFIA/ACIA 1524 (except the animal information section) including the signature block where the accredited veterinarian certified that he/she tested the animals described on date and at location shown.
- Attach to Form CFIA/ACIA 1524 a list of animals that were TB tested and found negative. In the animal information section of the Form CFIA/ACIA 1524, insert a statement that reads "The (insert # of animals) (insert species) listed on attached list were tested on the date shown above and at the location shown above with negative results". The accredited veterinarian should sign and date the list.
- Any animals with non-negative results must be included in the animal information section of Form CFIA/ACIA 1524.
- A copy of the completed Form CFIA/ACIA 1524 and the attached list are forwarded to the district office responsible of the area where the animals were tested. A copy must also accompany the export health certificate sent for endorsement to the district office (if different from the district where the animal was tested).
Comparative cervical tuberculin (CCT) test (for information purpose only. Not to be performed by accredited veterinarians)
68. It is recognized that the majority of screening test CFT/MCT reactions in Canada are not due to actual bovine TB infection. Follow-up comparative cervical tuberculin (CCT) testing serves to rule out the vast majority of false positive MCT reactions and identify any truly infected animals. The CCT test is performed by trained and qualified CFIA veterinarians.
69. The CCT test involves an intradermal injection of 0.1 mL of bovine PPD tuberculin and 0.1 mL of avian PPD tuberculin in the cervical region and is based on the premise that M. bovis (the agent of bovine TB) is somewhat antigenically distinct from M. avium and other related environmental mycobacterial species. The difference in size of reaction at both sites is used to determine if an animal's reaction to the CFT/MCT test was more likely due to exposure to M. bovis or to the antigens from M.avium or other cross-reactive mycobacteria. An animal exposed to M. bovis will have greater reactivity at the bovine PPD injection site relative to the avian PPD injection site.
70. Animals that are negative to the CCT test are not subject to further disease investigation activities.
71. Any animal that is classified as positive to the CCT test is considered to be a TB suspect and ordered destroyed (with compensation paid) for confirmatory testing. The animal is subject to a detailed post-mortem examination, followed by the laboratory examination of any abnormalities that may be observed, as well as a selection of normal tissues. If the laboratory analyses result in the isolation and identification of M. bovis, disease eradication activities are initiated on the affected premises.
72. For export purposes, although CFT/MCT reactor animals that are subsequently negative to the CCT test are not eligible for exportation, their herdmates with a negative CFT/MCT test result may be eligible for exportation. Contact the district veterinarian for more information.
73. For the CCT test to work properly, it must be applied at specific times after a screening CFT/MCT test, which in some circumstances requires a waiting period. Although this can be inconvenient for some individual producers, the delay is preferable to the possibility of missing infected animals which could spread disease. The CCT test may be applied as follows:
- up to 10 days following the injection of tuberculin for the CFT test (cattle and bison only);
- for the purpose of exportation to the USA only, the CCT test may also be performed on a cervid reactor to the MCT test up to 10 days following the injection of tuberculin for the MCT test, in order to qualify herdmates for export if the CCT is negative;
- at least 60 days following the injection of tuberculin for the CFT test (cattle, bison, goats, sheep); or
- at least 60 days following the injection of tuberculin for the MCT test (cervid).
All subsequent CCT test re-tests must be conducted at intervals of not fewer than 60 days.
Single post-axillary test for camelids
Preparation
74. A post-axillary intradermal tuberculin test is a standard test for tuberculosis in New World (llama, alpaca) and Old World (camels) Camelidae. Although this test is not validated for use in a domestic TB program, the post-axillary intradermal tuberculin test is performed in Canada under live animal export and import programs. Use the test procedures in this section, unless the export health certificate stipulates otherwise.
75. The test involves intradermal injections of 0.1 mL of bovine PPD tuberculin into the skin, posterior to the axilla and elbow of the animal, with observation, palpation, and measurement at 72 hours ± 6 hours post-injection.
76. Response to this test is determined by a relatively small change in skin thickness; therefore, it is imperative that a regimented approach is taken in applying the test. Measurements are influenced by the skin tension (related to animal positioning and restraint); by the amount of pressure applied to the skin by the callipers, and by the size of the skin fold picked up for measurement. Make every effort to standardize these conditions for both injection and reading.
77. Note: Do not apply this test to an animal that has had a tuberculin test of any kind during the preceding 90 days.
78. Ensure that this test is performed by a trained and qualified accredited veterinarian, subject to the requirements of the importing country.
79. Prepare the following equipment:
tuberculin – bovine PPD tuberculin (store in dark at 4°C)
syringes – 1 mL tuberculin syringes
needles – 26 gauge, ⅜" long
absorbent cotton
electric clippers with #40 head
callipers
Form CFIA/ACIA 1524 – Report of Intradermal Test
headlamp or flashlight, if poor lighting
80. Record the individual animal identification of each animal tested on Form CFIA/ACIA 1524. Acceptable animal identification is as follows: a HofA ear tag, a registered tattoo, or a microchip number, if a reader is available.
Injection
81. Adequately restrain each animal for testing to ensure correct animal identification, the proper preparation of the injection site, accurate pre-injection measurement, careful tuberculin injection, careful palpation, and accurate post-injection measurement.
82. Prepare the injection site by clipping an 8-cm square area on the lateral aspect of the chest wall in the axillary region near the point of the elbow.
83. Clean the injection site with cotton (moistened with water, if necessary). Do not use alcohol to clean the injection site. Inspect and palpate the intended injection site, recording any abnormalities, lesions, or scabs that are observed in the vicinity of the injection site on Form CFIA/ACIA 1524 to avoid being mistaken for tuberculin responses.
84. Prior to injecting the tuberculin, lift the fold of skin in the center of the injection area, apply the callipers to the fold, adjust the callipers just until they grip the skin but can still slip off the fold, slip the callipers off the fold, and read the calliper measurement to the nearest 0.5 mm. Record the measurement on Form CFIA/ACIA 1524.
85. Note: Perform a single calliper measurement at the injection site. Only if a problem is encountered with this measurement is the first measurement discarded and the entire process repeated. Repeated measurements will "flatten" the skin.
86. Draw 0.1 mL of bovine PPD tuberculin into the syringe. Inject the tuberculin intradermally into the center of the injection area, lifting the tip of the needle slightly after insertion to ensure it is clearly outlined under the skin. Inject 0.1 mL of tuberculin intradermally and withdraw the needle slowly.
87. View injection site to ensure that the injected tuberculin is visible and palpable as a definite bleb. If not, it indicates that either the needle was inserted too deeply or the dose was inadequate. In such a case, the administration of tuberculin should be repeated on the other side.
88. Discard needle immediately into a sharps container.
Reading
89. Observe and palpate the injection site in good light at 72 hours ± 6 hours after the injection to locate the point of greatest response. Note that, in camelids, response at the injection site is frequently diffuse. The response can also be highly variable.
90. Lift the fold of skin in the area of greatest response or, if there is no area of greatest response, in the centre of the injection area and measure the thickness of the fold with the same callipers used on the injection date and in the same manner as on the injection date, to the nearest 0.5 mm. Record the measurement to the nearest 0.5 mm, on Form CFIA/ACIA 1524.
91. Note: Perform a single calliper measurement at the injection site. Only if a problem is encountered with this measurement is the first measurement discarded and the entire process repeated. Repeated measurements will "flatten" the skin.
92. Subtract the pre-injection measurement from the post-injection measurement, and record the difference on Form CFIA/ACIA 1524. This value is the "skin thickness difference value."
93. Interpret the "skin thickness difference value," according to applicable export or import requirements. If interpretation is not specified, classify the animal's TB test result as follows and record on Form CFIA/ACIA 1524:
- Negative (Neg): There is no visible or palpable reaction at the injection site, and "skin thickness difference value" is less than 1.5 mm; or
- Reactor: There is a visible or palpable reaction at the injection site, or "skin thickness difference value" is equal to or greater than 1.5 mm. Contact immediately the CFIA district office.
94. All tuberculin tests must be recorded and reported to a CFIA District office by one of the following methods:
- Fully completed Form CFIA/ACIA 1524, including animal information for each animal tested. A copy of the completed Form CFIA/ACIA 1524 is forwarded to the district office responsible of the area where the animals were tested. A copy must also accompany the export health certificate sent for endorsement to the district office (if different from the district where the animals were tested); or
- Partially completed Form CFIA/ACIA 1524 as follows and attach an Animal List.
- Complete all parts of Form CFIA/ACIA 1524 (except the animal information section) including the signature block where the accredited veterinarian certified that he/she tested the animals described on date and at location shown.
- Attach to Form CFIA/ACIA 1524 a list of animals that were TB tested and found negative. In the animal information section of the Form CFIA/ACIA 1524, insert a statement that reads "The (insert # of animals) (insert species) listed on attached list were tested on the date shown above and at the location shown above with negative results". The accredited veterinarian should sign and date the list.
- Any animals with non-negative results must be included in the animal information section of Form CFIA/ACIA 1524.
- A copy of the completed Form CFIA/ACIA 1524 and the attached list are forwarded to the district office responsible of the area where the animals were tested. A copy must also accompany the export health certificate sent for endorsement to the district office (if different from the district where the animals were tested).
Base-of-the-ear tuberculin test for swine
Preparation
95. The base-of-the-ear intradermal tuberculin test is the standard test for tuberculosis in swine carried out under the Artificial Insemination Program and for the purposes of live-animal export.
96. Unless the importing country specifies different procedures and/or interpretation, use the procedures and interpretation set out in this section.
97. The test involves an intradermal injection of 0.1 mL of bovine PPD tuberculin into the skin at the base of the ear, with observation and palpation at 48 hours post-injection.
98. Note: Do not apply this test to an animal that has had a tuberculin test of any kind during the preceding 60 days.
99. Ensure that this test is performed by a trained and qualified accredited veterinarian, subject to the requirements of the importing country.
100. Prepare the following equipment:
tuberculin – bovine PPD tuberculin (store in dark at 4°C)
syringes – 1 mL tuberculin syringes
needles – 26 gauge, ⅜" long
absorbent cotton
Form CFIA/ACIA 1524 – Report of Intradermal Test
headlamp or flashlight, if poor lighting
101. Sufficiently restrain each pig for testing to ensure, correct reading of the animal identification, careful tuberculin injection, and proper reading of the test result. The method of restraint used can be modified according to the temperament and the weight of the pig. Avoid applying or observing the test without having the animal adequately restrained.
102. Record the individual animal identification of each animal tested on Form CFIA/ACIA 1524. Acceptable animal identification can be found under module 2.1 Identification of Livestock
Injection
103. The tuberculin injection site in swine is the dorsal surface of the ear, slightly anterior to the base. Use either the left or right ear, but be consistent.
104. Clean the dorsal surface of the base of the ear with cotton (moistened with water as necessary). Do not use alcohol to clean the injection site. Note any abnormalities, lesions, or scabs observed in the vicinity of the injection site, recording on Form CFIA/ACIA 1524 to avoid being mistaken for tuberculin responses.
105. Check tuberculin label to confirm type – M. bovis tuberculin has an orange label.
106. Check/confirm the tuberculin expiry date against the current date.
107. Disinfect rubber stopper with alcohol after opening aluminium seal.
108. Fill tuberculin syringe to 0.1 mL volume.
109. Standing to one side of the pig, insert needle into the dermis of skin at the base of the ear. Lift the tip of the needle slightly after insertion to ensure it is clearly outlined under the skin. Inject 0.1 mL of tuberculin intradermally and withdraw the needle slowly.
110. View injection site to ensure that the injected tuberculin is visible and palpable as a definite bleb. If not, it indicates that either the needle was inserted too deeply or the dose was inadequate. In such case, the administration of tuberculin should be repeated on the other ear.
111. Discard needle immediately into a sharps container.
Reading
112. Observe and palpate the injection site at 48 hours post-injection. Good lighting is essential.
113. Unless the importing country has stipulated a different interpretation, record the response to the test on Form CFIA/ACIA 1524 as follows:
- Negative (Neg.): There is no visible or palpable change in tissue at the site of injection; or
- Reactor: There is an appreciable visible or palpable disturbance in tissue at the site of injection. Notify the CFIA district office.
114. No ancillary comparative skin testing is available. Whenever a porcine animal is classified as a reactor, an investigation must be undertaken by CFIA to identify any contact that the reactor may have had with ruminant animals since its birth. If any such contact is identified, the ruminant animal herd must be investigated and tested by CFIA employees.
115. The fate of a reactor porcine animal is determined by the standards of the program under which the animal was tested, and may include retesting the reactor after at least 60 days or removing the animal from the semen production centre or export shipment.
116. All tuberculin tests are official and must be recorded and reported to a CFIA district office by one of the following methods
- Fully completed Form CFIA/ACIA 1524 including animal information for each animal tested. A copy of the completed Form CFIA/ACIA 1524 is forwarded to the district office responsible of the area where the animals were tested. A copy must also accompany the export health certificate sent for endorsement to the district office (if different from the district where the animals were tested) or the certificate of health for entry into a semen production centre (Form CFIA/ACIA 1634 or isolation release document); or
- Partially completed Form CFIA/ACIA 1524 as follows and attach an Animal List.
- Complete all parts of Form CFIA/ACIA 1524 (except the animal information section) including the signature block where the accredited veterinarian certified that he/she tested the animals described on date and at location shown.
- Attach to Form CFIA/ACIA 1524 a list of animals that were TB tested and were found. In the animal information section of the Form CFIA/ACIA 1524, insert a statement that reads "The (insert # of animals) (insert species) listed on attached list were tested on the date shown above and at the location shown above with negative results". The accredited veterinarian should sign and date the list.
- Any animals with non-negative results must be included in the animal information section of Form CFIA/ACIA 1524.
- A copy of the completed Form CFIA/ACIA 1524 and the attached list are forwarded to the district office responsible of the area where the animals were tested. A copy must also accompany the export health certificate sent for endorsement to the district office (if different from the district where the animals were tested) or the certificate of health for entry into a semen production centre (Form CFIA/ACIA 1634 or isolation release document).
3.2 Serologic Testing
This part of the Accredited Veterinarian's Manual will provide the following information necessary for serologic testing:
- collection, material and supplies
- preparation and packaging of serum samples
- laboratories
- submission forms
- paper submissions
- digital submissions with a Canadian Food Inspection Agency-approved digital Equine Infectious Anemia (EIA) certification system
- special procedure for submission to the CFIA laboratories, applicable to samples from bovine tested in isolation (IAI notification), from sheep and goats, wild boar or cervids for export to Mexico and bovines tested under the Canadian Health Accredited Herd – Enzootic Bovine Leukosis (EBL-CHAH) program
- shipping samples
- criteria for rejection of samples and submission forms in CFIA animal health laboratories
- test results
Objectives
Section 2 of the Health of Animals Regulations interprets "test" as including:
- the collection of body tissue or fluid from an animal (for example, serum)
- the injection of an animal for the purpose of determining that animal's freedom from infection with disease (for example, tuberculin testing)
The reliability of any diagnostic laboratory procedure depends directly on the type of specimens or samples received and on their condition. The laboratory must receive a sample of good quality for diagnostic purposes.
Collection, materials and supplies
It is extremely important that animals are identified correctly. Animals which are tested must be uniquely identified using a approved indicator for the species being tested. Where no approved indicator exists for species tested, refer to Module 2.1 Identification of Livestock or Module 2.2 Identification of horses for more information.
Unless otherwise indicated, a minimum of a 1 ml sample of serum is required for each test.
- if using serum separator tubes (SSTs), ensure that they are centrifuged at a high enough g-force (1100-1300 relative centrifugal force) to properly seat the separating gel.
- After centrifugation, decant the serum from the SST into a new red top tube before shipping to the laboratory.
- the centrifuged SST tube alone will NOT prevent hemolysis and leakage of blood cell components back across the plug into the serum fraction.
A separate sample must be submitted for each diagnostic test required even if various tests are requested and are done at the same laboratory. Laboratories prefer separate serum samples for each test in order to maintain sample integrity. For example, if brucellosis and leptospirosis tests are requested, 2 samples are required.
For clotted blood samples:
- use silicone coated tubes (red stopper), which should be centrifuged before shipping and the clot removed
- serum should be kept refrigerated until shipment
- use disposable needles
- use a waterproof marker to number the vials
Small (3 ml) vials with or without internal thread closures should be avoided as these are prone to leakage and may cause technical problems for the laboratory.
The costs associated with material and supplies must be paid by the accredited veterinarian.
Preparation and packaging of serum samples
Each vial must be marked indelibly with the number corresponding to the one entered on the submission form in the column under Vial No. Label the vial, not the stopper.
Paired samples must be identified with the same vial number for the same animal.
- the acute sample (the oldest one) will be identified A, and the convalescent sample (the most recent) will be identified C (for example: 001A, 001C, 002A 002C, 003A, 003C, etc.)
Accredited veterinarians must make sure that the packaging and shipping procedures used complies with regulations concerning the transportation of such samples.
- the majority of samples collected under the Accredited Veterinarian Program will be classified as "Exempt Animal Specimens" under the Transportation of Dangerous Goods Regulations
- Exempt Animal Specimens are defined as "Animal specimens not believed to contain infectious substances (for example: samples taken for surveillance or export purposes) or specimens where the pathogens has been neutralized/inactivated"
- for samples not meeting this definition, consult with you District Veterinarian as to the proper classification and shipping procedures required
- refer to the figure below for the standards required when shipping Exempt Animal Specimen
Outer package markings checklist
- name, address, phone number of the shipper and consignee
- the words – "Exempt Animal Specimen" on the packaging
Statements on Shipper's Waybill
- statement – Exempt Animal Specimen
Note: if multiple fragile primary receptacles are placed in a single secondary packaging, they must either be individually wrapped or separated to prevent contact between containers.

Long description
Pictorial representation of the minimum packaging shipping standards required for Exempt Animal Specimens
Outer packaging
Secondary packaging leakproof or siftproof (for example sealed plastic bag)
Primary receptacle leakproof or siftproof
Specimen
Absorbent packing material (for liquids)
Vials should be packed in boxes designed for this purpose in a manner to prevent breakage (for example, a New York box or styrofoam vial shipper).
- the use of an appropriate container is a requirement when submitting samples to a laboratory
- in addition to a legal responsibility, a submitter has a moral obligation to ensure the safety of transportation industry workers and laboratory staff handling the specimen
The laboratory submission forms should be placed in an envelope and attached to the outside of the protective containers.
Laboratories
Most testing, mandated by the Health of Animals Act and regulations or conducted in response to a specific request by an importing country, is conducted in a CFIA laboratory or a CFIA-approved laboratory, unless specified otherwise.
Certain private laboratories are specifically approved by the CFIA to conduct official tests for Equine Infectious Anemia (EIA) and Brucellosis submitted by accredited veterinarians.
- in such cases the accredited veterinarian manual will specify that the test may be performed in a private or authorized laboratory that meets the requirement of the Policy on the Use of External Laboratories
- the accredited veterinarian must verify with the CFIA district office that the laboratory meets the specified requirements before sending a sample
The accredited veterinarian is responsible for the laboratory fees.
- applicable fees for test submitted to CFIA laboratories are available in Module 4.2 CFIA Fees
All specimens sent to a CFIA or CFIA-approved laboratory must be submitted with a CFIA-approved submission form for the official test being requested.
- please contact the CFIA-approved laboratory directly to confirm what documentation or notification is required when using a CFIA-approved digital EIA certification system for sample submission. (Contact information available in Module 3.3)
Paper submission forms
Submission forms for samples sent to CFIA laboratories are available at the CFIA district office.
- for samples submitted to a CFIA laboratory, use Form CFIA/ACIA 5473 – Animal Health Import, Export and Artificial Insemination Specimen Submission
- instructions for submission of samples are printed on that form
- a laboratory notification number, which is available from a CFIA district veterinarian, must be entered in the "Reason for Test" section of Form CFIA/ACIA 5473
- ensure the correct notification number for the samples being tested is used
- for each test requested, a serum sample and a copy of the appropriate completed submission form must be sent to the laboratory
- a copy of the submission form must be kept on file and a copy must be sent to the CFIA district office when the sample is submitted to a CFIA laboratory
To submit brucellosis samples to a CFIA-approved laboratory, use Form CFIA/ACIA 5473 as well.
- when samples are tested for export purpose, a notification number is not required on Form CFIA/ACIA 5473, but the destination should be identified as "USA or Mexico"
it is acceptable to complete the first 2 pages of Form CFIA/ACIA 5473 in full and mark the third page with "See attached list"
The list can be a spreadsheet that records the animals' identification, description and the vial numbers, and should identify "form reference #_______________" and be signed.
- the form reference number is the number which appears in the bottom centre of all 3 pages of Form CFIA/ACIA 5473 and must begin with the current calendar year
- it should be noted that each CFIA Form 5473 bears a unique reference number and can only be used once
- Do not make photocopies of these forms for later use
Note: for brucellosis samples collected for purposes other than export or the artificial insemination program (for example pre movement of zoological species), please consult with the local CFIA District Office for proper submission procedures.
To send EBL-CHAH samples to a CFIA laboratory, use Form CFIA/ACIA 3841 – Canada Health Accredited Herd – Serum Test Report.
- an electronic template for submission of EBL-CHAH samples must be used in lieu of filling out the Animal Information Table of the CFIA 3841 (see below)
For accredited veterinarians working for clinics who have applied for and been granted a "digital exemption" (refer to Module 8.4 Equine infectious Anemia), EIA samples can be sent to a CFIA-approved laboratory using Form CFIA/ACIA 3937 – Equine Infectious Anemia (EIA) Serum Test Report and Certificate.
Forms should be typed or printed and all copies must be legible.
- all information should be recorded on the form including ear tag numbers, tattoos or other types of identification
- the type of test being requested and the date and location where the specimens were collected should be recorded
- accredited veterinarians must include their name, address (including postal code) and telephone number, and must sign at the bottom of the form
- the submitter code must also be indicated, as well as the identification code of the CFIA district office in which the animals tested are located
It is the responsibility of the accredited veterinarian to provide all information required.
- laboratories may refuse to conduct analysis of samples submitted with forms that are not fully complete
- please refer to a CFIA district veterinarian for examples of completed forms and for instructions on the distribution of forms for samples being sent to CFIA laboratories
Digital submissions using a CFIA-approved digital EIA certification system
For accredited veterinarians working for clinics who have not been granted a "digital exemption", a CFIA-approved digital EIA certification system must be used to submit samples for EIA testing to a CFIA-approved laboratory.
- data fields of the electronic system are based on Form CFIA/ACIA 3937
- for certification system provider contact information, please refer to the List of CFIA-approved Electronic EIA Certification Systems in Module 8.4 Equine infectious Anemia
The accredited veterinarian must:
- register with the service provider and receive the necessary training
- send samples to a CFIA-approved laboratory which is registered with the same provider (laboratory contact information available in Module 3.3)
For more details on the required information and digital photographs for identification, please refer to Module 8.4 Equine Infectious Anemia.
Accredited veterinarians are responsible for the security and proper use of their digital signature and shall take reasonable care to prevent its misuse.
only accredited veterinarians are authorized to sign documents using their password protected digital signature
Other clinic staff does not have the authority to be part of the signing process. Utilization of your digital signature by anyone other than yourself would be considered a breach of your agreement as an accredited veterinarian and would render the form invalid.
- please note that provisions of article 10 from your Accredited Veterinarian Agreement also apply to the digital submission of documents through an digital system
Please contact the CFIA-approved laboratory directly to confirm what documentation or notification is required when using a CFIA-approved digital EIA certification system for sample submission. (Contact information available in Module 3.3).
Final CFIA-approved digital EIA test certificates are recognized as official EIA test results and can be accessed online in real-time by the owner (optional), the accredited veterinarian, the CFIA-approved laboratory and CFIA.
- Black and white or colour printed copies of the electronic EIA test certificate are both acceptable and can be used for domestic use within Canada and as proof of a negative EIA test result for export purposes
For additional information regarding the use of an approved digital EIA certification system, please contact the CFIA district office.
Special procedure for submission to CFIA laboratories
This section is applicable to samples from bovine tested in isolation (IAI notification), from sheep and goats, wild boar or cervids for export to Mexico and from bovine tested under the EBL-CHAH program
When an accredited veterinarian needs to send samples to be analyzed in CFIA laboratories from bovine under isolation notification (IAI), sheep and goats, wild boar or cervids for exportation to Mexico or bovines under the EBL-CHAH program, an Excel spreadsheet must be completed and forwarded electronically in addition to the paper form submission (CFIA Form 5473 or CFIA Form 3841) that will be attached to the samples to be sent.
- The template for each activity listed above is available through your local CFIA district office
The spreadsheet will be used by the CFIA laboratories to import data into the Laboratory Sample Tracking System (LSTS), preventing transcription errors and saving time, especially on larger submissions.
The template must be completed as follows:
- for each submission, a new template will need to be completed
- make sure to save it under a new name containing the reference number of the CFIA Form 5473 form it will refer to (for example 2014IEAB-0000079706-4)
- for EBL-CHAH submissions, the file name should contain the name of the herd sampled and date of testing
- the properly named Excel file must be sent to a generic email address: cfia.labtemplate-gabaritlab.acia@inspection.gc.ca
- a copy must be sent as well to the local district office where the animals were located when tested
- each email must contain only 1 attached template
- in the email subject line, provide the CFIA Form 5473 reference number or name of the EBL-CHAH herd only
- in the body of the message, you may provide info on the date the samples are shipped, if possible
no column can be deleted or added to the template and the mandatory format is to be followed
Otherwise, the importation of data at the laboratory level will fail leading to a delay in the analysis until a new template is forwarded.
- mandatory fields in the template include sample No., date sampled, sample type, animal identification (ID) (at least 1 of the 6 fields about ID is mandatory) and breed
- enter the age in column N
- if the age is unknown this column may be left blank as long as you select from the drop down menu "estimate" for column L and "not provided" for column M
- some fields do not apply for isolation testing or for export to Mexico
- They can't be deleted but may be left blank.
The generic laboratory email address is not assigned to a specific laboratory and cannot be used to submit questions or requests
- Any questions will need to be directed to the local CFIA district office
The electronic submission of the template does not preclude the requirement to send a paper copy of the submission form along with the sample.
- it is acceptable to complete the submission form in full and mark "See attached list" in the animal information table
- the list can be a copy of the electronic spreadsheet that records the vial numbers, the animal's identification, identify the "form reference #_______________" and be signed
- the reference number is the number which appears in the bottom centre of all 3 pages of Form CFIA/ACIA 5473
- to be acceptable, the attached list must show all the information for each animal on the same page (use the hide function from the spreadsheet to remove unnecessary columns), the reference number must be written on every page, and each page needs to be initialed by the accredited veterinarian
Shipping samples
Include all pertinent/required forms with the samples.
- Please contact the CFIA-approved laboratory directly to confirm what documentation or notification is required when using a CFIA-approved digital EIA certification system for sample submission. (contact information available in Module 3.3)
The shipment of samples must never be entrusted to the animal owner or exporter.
- the accredited veterinarian must be able to maintain a chain of custody for samples shipped to the laboratories
- samples to be shipped may be grouped at an intermediary location into a larger shipment provided that the samples are not opened and / or repackaged after leaving the accredited veterinarian possession
After collection, samples are refrigerated and shipped to the laboratory without delay. Use the safest and quickest means of shipment available.
Ship samples only during the week to avoid having them delayed in transit or held over at the laboratory on weekends or holidays.
- it is advised to only ship samples Mon-Wed, unless receipt at the lab can be guaranteed prior to close of business for the weekend
- pay particular attention to holiday closures
- in case of delayed shipment, serum samples for EIA may be frozen and sent at a later date
- consult Module 8.4 Equine Infectious Anemia for detailed instructions regarding storage and timing of analysis to ensure validity of results
- Samples for virus isolation tests such as Bovine viral diarrhea – Immunoperoxidase (BVD-IP) must not be frozen prior to submission, and must be protected from extremes of temperature during transit
- any factor that affects the viability of the agent in question will affect the sensitivity of the test and therefore the reliability of the test result
Vial breakage will require re-bleeding an animal and significantly prolong laboratory test times.
It is the responsibility of the accredited veterinarian to resubmit if a specimen is unfit for testing. Refer to the tables below for common causes of "unfit" samples.
The accredited veterinarian is responsible for shipping costs.
- if samples are sent by mistake to the wrong CFIA laboratory, the laboratory may as a courtesy resend it to the appropriate laboratory, if the accredited veterinarian has agreed to provide their courier account number to cover the shipping cost
Criteria for rejection of samples and submission forms in CFIA animal health laboratories
CFIA and CFIA-approved labs must adhere to quality assurance standards in the receipt and testing of all samples received. CFIA-approved labs set their own internal criteria for the rejection of samples received, and have protocols in place to notify the submitters of samples rejection and the reasons for the rejection. These criteria can be obtained directly from the specific CFIA-approved lab. For samples submitted to CFIA labs, criteria for rejection are listed in the tables below.
Reasons for rejection may be related to the sample itself or the sample submission form. Notification of rejection of samples will come to the accredited veterinarian through the CFIA District Office and will consist of the submission reference number, vial(s) number(s) of the rejected samples (if applicable) and the reason for rejection. Based on the reason for rejection, the accredited veterinarian will be required to undertake the appropriate corrective measures for the sample(s) and/or the submission form in order to complete the testing.
A – General for all sample types
| No. | Criteria of rejection for samples | Result on Record of Analysis (ROA) | Comments |
|---|---|---|---|
| A1 | Contamination | Unfit |
|
| A2 | Incorrect type of sample or container | Unfit |
|
| A3 | No ID on sample's container (vial/bag) | Unfit |
|
| A4 | Error in ID | Unfit |
|
| A5 | Duplicate samples | Unfit |
|
| A6 | Missing sample | No sample received (NSR) |
|
| A7 | Extra sample | Sample is not tested |
|
| A8 | Broken vial | Broken on arrival |
|
| A9 | Insufficient volume/quantity | Insufficient sample |
|
| A10 | Sets of samples with the same IDs in a unique box | Unfit |
|
B – Serology samples
| No. | Criteria of rejection for samples | Result on Record of Analysis ROA | Comments |
|---|---|---|---|
| B1 | Lipaemia | Unfit |
|
| B2 | Haemolysis | Unfit |
|
| B3 | Fibrin clot (avian samples) | Unfit |
|
C – Bovine Spongiform Encephalopathy (BSE) samples
Note: BSE testing is not done under the Accredited Veterinarian Program, but accredited veterinarians may be involved in the collection of these sample on behalf of CFIA
| No. | Criteria of rejection for samples | Result on Record of Analysis ROA | Comments |
|---|---|---|---|
| C1 | Damaged sample | Not tested – No sample |
|
| C2 | Autolyzed sample | Not tested – Unsuitable |
|
| C3 | Incorrect type of sample | Not tested – No sample |
|
D – Other specific sample types
Note: these specifications apply to samples taken for routine tests and are an excerpt from the document Animal Health Tests and Analyses performed in CFIA laboratories
| No. | Criteria of rejection for samples | Result on Record of Analysis ROA | Comments |
|---|---|---|---|
| D1 | Avian Influenza | Unfit |
|
| D2 | Avian Paramyxovirus (Newcastle Disease) | Unfit |
|
| D3 | Bovine Viral Diarrhea | Unfit |
|
| D4 | Campylobacter fetus | Unfit |
|
| D5 | Contagious Equine Metritis | Unfit |
|
| D6 | Cysticercosis | Unfit |
|
| D7 | Elaphostrongylus cervi | Unfit |
|
| D8 | In-vitro fertilized embryo – virus isolation | Unfit |
|
| D9 | Infectious Bovine Rhinotracheitis | Unfit |
|
| D10 | Mycoplasma | Unfit |
|
| D11 | Salmonella | Unfit |
|
| D12 | Tritrichomonas (Trichomonosis) | Unfit |
|
| D13 | Turkey Rhinotracheitis (TRT) | Unfit |
|
| D14 | All Histopathology tests (for example Cysticercosis, TB) | Unfit |
|
Test results
The accredited veterinarian is responsible for advising the owner of the results.
Test results reported from CFIA laboratories are generated through the Laboratory Sample Tracking System (LSTS).
- the test results will be provided to the accredited veterinarian by the local district office
Test results from CFIA-approved laboratories are usually reported directly to the submitter and the CFIA district office.
For additional information on distribution of test results when using a CFIA-approved electronic EIA certification system, please refer to Module 8.4 Equine Infectious Anemia.
Test results from private laboratories are usually reported directly to the submitter only. The accredited veterinarian will provide a copy of such test results to CFIA upon request.
Schedule testing so as to allow sufficient time to receive results.
- turn around times for samples tested are relative to the day the samples are received by the laboratory.
3.3 Laboratories
Laboratories approved to test EIA and brucellosis – Buffered plate antigen test (BPAT)
Updated:
December 2022
| Approved laboratory | EIATable Note 1 | Bruc. |
|---|---|---|
| Animal Health Center 1767 Angus Campbell Rd. Abbotsford, B.C. V3G 2M3 Tel: 604-556-3135 Fax: 604-556-3133 Contact: Tracy Roddick e-mail: tracy.roddick@gov.bc.ca Lab e-mail: serology@gov.bc.ca Contact QA: Liliana Camargo e-mail: liliana.camargo@gov.bc.ca |
YesTable Note 2 | YesTable Note 2 |
| Animal Health Laboratory University of Guelph 419 Gordon Street, Bldg 89 Guelph, Ontario, N1G 2W1 Lab phone: 519-824-4120 ext. 54514 Virologist (Dr. Davor Ojkic) Phone: 519-824-4120 ext. 54524 Fax: 519-821-8072 Lab e-mail: ahl.virology@uoguelph.ca Virologist email: dojkic@uoguelph.ca |
Yes | No |
| Antech Diagnostics (Mississauga facility) 7555 Danbro Crescent Mississauga, ON, L5N 6P9 Tel: 905-567-0597 Fax: 905-567-9021 Contact lab: Kathy Zito e-mail: kathy.zito@antechmail.com Contact QA: Gregory Freeman e-mail: gregory.freeman@antechmail.com |
Yes | No |
| Atlantic Veterinary College Regional Diagnostic Virology Laboratory 550 University Avenue Charlottetown, P.E.I. C1A 4P3 Tel: 902-566-0877 Fax: 902-566-0723 Contact lab: Dr. Carmencita V. Yason Phone: 902-566-0752 e-mail: yason@upei.ca Contact QA: Jocelyn Phillips |
Yes | No |
| Biovet Inc. 4375 Avenue Beaudry St-Hyacinthe, Québec J2S 8W2 Tel: 450-771-7291 Fax: 450-771-4158 Contact: Amélie Francoeur, Mcb. A. ext : 971848 e-mail: amelie.francoeur@biovet-inc.com Contact lab: Annie St.-Germain Lab: ext 971888 Fax: 450-771-2996 e-mail: annie.st-germain@biovet-inc.com Contact QA: Annie Blier e-mail: annie.blier@biovet-inc.com |
Yes | Yes |
| Centre de diagnostic vétérinaire de l'Université de Montréal 3200 Sicotte, St-Hyacinthe, QC, J2S 2M2 Tel: 450-773-8521 ext 8534 Fax: 450-778-8107 Contact AQ: Véronique Bournival e-mail: veronique.bournival@umontreal.ca |
Yes | No |
| Idexx Reference Laboratories (Markham) 1345 Denison Street Markham, ON L3R 5V2 Tel: 800-667-3411 Fax: 905-475-7309 Contact lab: Grace Rejano Ext. 86288 Tel: 905-968-6288 e-mail: Grace-Rejano@idexx.com Contact QA: Meghann Bryan e-mail: Meghann-Bryan@idexx.com Other contact: Paul Burton e-mail: Paul-Burton@idexx.com |
Yes | Yes |
| Prairie Diagnostic Services Inc. 52 Campus Drive Saskatoon, SK, S7N 5B4 Fax.: 306-966-7737 Contact lab: Anju Tumber Phone: 306-966-7223 e-mail: anju.tumber@pds.usask.ca Contact QA: Karen Moline Phone: 306-966-7952 e-mail: karen.moline@pds.usask.ca |
Yes | Yes |
| True North Veterinary Diagnostics Inc. 6325 204th Street, Unit 320 Langley, B.C., V2Y 3B3 Phone: 604-539-5550 Fax: 888-336-9408 Contact lab: Maria Neukamm e-mail: marianeukamm@hotmail.ca Other contact: Dr. Allan Berrington e-mail: allan.berrington@truenorthvet.ca |
Yes | No |
| Veterinary Diagnostic Services 545 University Crescent Winnipeg, Manitoba R3T 5S6 Tel: 204-945-7185 Fax: 204-948-2654 Contact for BRUC: Cheryl Friday e-mail: Cheryl.Friday@gov.mb.ca |
No | Yes |
Canadian Food Inspection Agency (CFIA) laboratories
CFIA's Animal Health Laboratory Services are delivered by a network of Centres of Expertise, each of which is the national centre for its area of specialization.
The Centres are located at the following CFIA laboratories:
CFIA Lethbridge Laboratory
(Animal Diseases Research Institute)
225090 Township Road 91
Lethbridge County, Alberta T1J 5R7
Telephone: 403-382-5500
Facsimile: 403-381-1202
- Bovine and Equine Indigenous Viral Diseases Centre of Expertise
- Anthrax and Leptospirosis Centre of Expertise
- Non-traditional Livestock Centre of Expertise
- World Organisation for Animal Health (WOAH; founded as Office International des Épizooties (OIE)) Reference Laboratory for Anthrax, BSE and BVD
CFIA Saskatoon Laboratory
(Centre for Food-borne and Animal Parasitology)
116 Veterinary Road
Saskatoon, Saskatchewan S7N 2R3
Telephone: 306-385-7824
Facsimile: 306-385-7866
- Parasitic Diseases of Livestock and Farmed Game Animals Centre of Expertise
- WOAH Reference Laboratory for Trichinellosis
- WOAH Collaborating Centre for Food-Borne Zoonotic Parasites
CFIA Winnipeg Laboratory
(National Centre for Foreign Animal Disease, Canadian Science Centre for Human and Animal Health)
1015 Arlington Street
Winnipeg, Manitoba R3E 3M4
Telephone: 204-789-2001
Facsimile: 204-789-2038
- WOAH Reference Laboratory for AI and CSF
CFIA Ottawa Laboratory
3851 Fallowfield Road
Ottawa, Ontario K2J 4S1
Telephone: 343-212-0500
Facsimile: 343-212-0204
- Avian Diseases Centre of Expertise
- Brucellosis Centre of Expertise
- Germplasm Centre of Expertise
- Mycobacterial Diseases Centre of Expertise
- Rabies Centre of Expertise
- WOAH Reference Laboratory for Rabies, Scrapie and CWD
CFIA Saint-Hyancinthe Laboratory
(Health of Animals and Food Laboratory)
3400 West Casavant boulevard
Saint-Hyancinthe, Quebec J2S 8E3
Telephone: 450-768-6800
Facsimile: 450-768-6767
- Indigenous Porcine Diseases Centre of Expertise
- Retrovirology Centre of Expertise
3.4 Testing for bovine genital campylobacteriosis and trichomonosis
This part of the Accredited Veterinarian's Manual will provide the following information necessary for testing for campylobacteriosis and trichomonosis:
- general information
- materials and supplies
- sample collection
- preparation and packaging
- submission forms
- shipping
- laboratories
- test results
- laboratory fees
Objectives
1. Section 2 of the Health of Animals Regulations interprets "test" as including:
- the collection of body tissue or fluid from an animal (e.g. serum, preputial smegma);
- the injection of an animal for the purpose of determining that animal's freedom from infection with disease (e.g. tuberculin testing).
2. The sampling procedure described in this section may be used to submit samples for export purposes (for example, to qualify cattle to Mexico when applicable) or to meet test requirements for the National Artificial Insemination Program.
General information
3. Tritrichomonas foetus (also referred as Trichomonas foetus or T. foetus) is a flagellated protozoan parasite that lives in the reproductive track of cattle, and is only transmitted through venereal contact. Bulls that become infected tend to become carriers for life, and they are the main reservoir for the disease. In bulls, the organism is found in the crypts of the penile or preputial mucosa. Tritrichomonas foetus may be sparsely and unevenly distributed in the preputial cavity. The highest concentration of the organism is thought to occur at the glans penis. Cows that become infected may experience embryonic death, abortion, or pyometra. Some cows may remain infected throughout a successful gestation and transmit the infection at the start of the next season. Although the prevalence of this carrier cow phenomenon is unknown, it is believed to be low.
4. Campylobacter fetus is divided into 2 subspecies: C. fetus subsp. venerealis and C. fetus subsp. fetus. By definition, C. fetus subsp. venerealis is the cause of Bovine Genital Campylobacteriosis (BGC), a venereally transmitted disease of cattle causing infertility, early embryonic death and abortion with considerable economic losses in endemic regions. While bovine infections with C. fetus subsp. fetus can also occur, they are only associated with sporadic abortion. C. fetus ssp. venerealis is the agent of interest for the National Artificial Insemination Program and the export certification for bovines to Mexico.
Materials and supplies
5. The costs associated with collection materials, packaging and shipping charges for transporting samples must be paid by the accredited veterinarian.
Material required:
- disposable gloves
- scissors
- 53 cm (up to 68 cm in larger bulls) infusion/insemination pipette, individually wrapped
- disposable 20 ml syringe
- disposable 3 ml syringe with small gauge needle (not greater than 20G)
- collection and transport media for each organism:
Each organism (Campylobacter and Tritrichomonas) has specific transport media requirements. The transport and collection media itself is provided at no charge at the moment. However, there is a shipping fee for transporting the media from the laboratory to the requesting client (accredited veterinarian or artificial insemination centre). A courier service account number must be provided when ordering the material- Campylobacter: transport enrichment medium (TEM) and saline vials are ordered from the Animal Health Microbiology Laboratory located at the CFIA Ottawa Laboratory (Fallowfield). A specific order form is available through the district office. TEM transport and liquid sample collection (saline) vials are shipped from the laboratory together in one foam container covered by a corrugated cardboard sleeve and placed in a larger polystyrene foam chest with ice packs. If the TEM vials happen to warm up during transport to the clinic, this is not critical for their subsequent use. However, once received, the TEM and collection vials must be stored at 4°C until use. The foam container carries 10 TEM transport vials and 6 or 7 saline collection vials. Duplicate TEM vials are required for each animal tested. The outer sleeve on each foam container holds the unit together and has red arrows indicating the UP position of the vials. Slip the cardboard sleeve off the foam container (the top of each foam container is stamped in both English and French). Remove the top from the foam container to reveal both the TEM and saline collection vials. Check for a media label (attached to the outside of each vial) and the expiry date which is written on the media label. All TEM vials must have a media label and be inoculated on or before the expiry date to be accepted for culture. TEM vials have a shelf life of 90 days from date of production.
- Tritrichomonas foetus transport media (TFTM): the transport media is sent from the centre for Food-borne and Parasitology at the CFIA Saskatoon Laboratory. A specific order form is available through the district office. The form must be faxed to the laboratory to order the media. The transport media must be stored at 4°C as well until use and has a shelf life of 14 days from date of production. Media can be used up to, and including, the date of expiry.
Sample collection
6. Good sample collection technique as well as care in storage and shipping are very important to achieve the highest possible sensitivity. For sampling, all transport media (TEM and TFTM) and saline vials should be brought to room temperature (18-37°C) prior to inoculation with sample. Expiry date must be verified before sampling procedure begins. A video (DVD) produced by the CFIA Saskatoon Laboratory is available at the district office to view the collection procedure for Tritrichomonas sampling.
7. Two separate samples should be collected if testing for both organisms is required.
8. The animal must be restrained to avoid injury to the collector and to the animal. Separate sampling materials must be used for each animal collected.
- Male:
- Preputial fluid (smegma) is collected. Sampling should not be conducted until at least 7 days have elapsed following natural service or semen collection by means of an artificial vagina.
- Use a separate pair of disposable gloves for each animal. Long hairs and fecal balls at the prepuce orifice should be cut with scissors to reduce contamination. Care must be taken to properly disinfect and rinse the scissors between different animals. Washing the prepuce is not advisable as this may actually introduce contaminants and/or flush out available organisms. Disinfectant must be avoided. If the preputial area is excessively dirty, the external part only can be carefully cleaned with water and dried with paper towel.
- Insert the pipette into the prepuce about half way. Connect a 20 ml syringe to the pipette and apply negative pressure. The pipette should be held parallel along the length of the prepuce. In situations where introducing contamination into the sheaths is a concern, the plastic cover of the pipette may be left in place until the end of the pipette extends beyond the dirtiest part of the preputial orifice. Advance the pipette until it reaches the fornix. Using back and forth movements from the fornix to within 5 cm of the preputial orifice, slide the pipette 10-15 times to gently scrape the preputial mucosa. It is important that the pipette contacts the glans penis. The scrapings will be collected by the negative pressure in the pipette. Retract the pipette and syringe.
- Female animals:
- Cervical mucus or vaginal discharge must be sampled.
- Use a separate pair of disposable gloves for each animal. Debris should be cleaned from the vulva with dry paper towel. No disinfectant should be used.
- Immobilize the cervix per rectum and insert the pipette in the cranial third of the vagina. The tip of the pipette is advanced to the cervix. Negative pressure is applied to collect cervical mucus via a syringe. Vaginal discharge may also be collected from the anterior vagina. Retract the pipette and remove it.
Sample preparation and packaging instructions
9. The reliability of any diagnostic laboratory procedure depends directly on the type of specimens or samples received and on their condition. It is extremely important that animals are identified correctly and the information entered on the forms is complete and accurate.
10. Each vial must be marked indelibly with the number corresponding to the one entered on the submission form in the column under Vial # for the corresponding animal being tested. Duplicate vials for Campylobacter must be identified with the same vial number for the corresponding animal.
11. Tritrichomonas:
- Properly label the TFTM vial. Expel all collected sample material into the TFTM media. Gently aspirate some transport media into the pipette, and repeatedly rinse any remaining contents of the pipette and syringe into the media.
- Transport media for Tritrichomonas foetus are sent in an insulated container that should be reused to send the samples back to the laboratory. Place TFTM samples back into the box from which they originated. Inoculated material must be packed to exclude light and temperature fluctuation. To prevent temperature fluctuation during shipping, samples placed in insulated container should be packed with newspaper or alternative insulating material to fill any air space. Samples must be kept between 10-37°C. When the samples are stored or shipped at 4°C or below, the viability of the organisms decreases and thus the sensitivity of the test decreases. Use of insulated containers is essential. Samples frozen or shipped on ice will not provide reliable results and will require re-collection. In summer months, care should be taken to make sure that the samples are not exposed to temperatures exceeding 39°C. Samples must reach the Saskatoon laboratory within 5 days of collection or they will not be tested and will be reported as "Unfit".
12. Campylobacter:
- Properly label 2 TEM vials and one saline vial. Uncap the saline collection vial. Collect a preputial sample as per previous instructions and transfer the sample into the saline vial by submerging the collection pipette tip into the liquid and expel any material collected in the pipette and syringe. Repeatedly rinse any remaining contents by pulling back and forth the syringe plunger very gently several times in the vial.
Note: the sample must not be left in the saline for more than 15 minutes before the transfer and inoculation of the TEM vials. - Using a 3 ml disposable sterile syringe and a small gauge needle (should be no larger than 20 gauge), carefully aspirate 2 ml of the upper layer from the saline collection vial. Exclude all air from the syringe before transfer. TEM vials are provided with a piece of adhesive tape over the top. Lift the free end of the adhesive tape to expose the rubber membrane in the top (Do not unscrew the top of the TEM vials as this will disrupt the special gas mixture within).
- Inject 1 ml of the aspirated saline (step b. above) through the rubber membrane into the TEM vial. Replace the adhesive tape over the rubber membrane. Do not inject the aspirated sample directly through the adhesive tape.
- Repeat this step for the second TEM vial using the remaining 1 ml aspirated saline in the syringe. Mix the contents by gently shaking the TEM vials.
- Place the TEM vials and used saline collection vials back into their placement slots in the bottom portion of the foam container from which they originated. When all slots in the foam container are filled with the TEM transport and saline vials, place the foam top on the container, and slide the corrugated sleeve over the unit with the red arrows pointed up.
- Place the foam containers into a larger polystyrene foam chest without ice packs (temperatures during transport should be between 18-37°C) and ship back to the CFIA Ottawa laboratory (Fallowfield) without delay. Use the safest and quickest means of shipment available and ship samples only during the week to avoid having them delayed in transit or held over at the laboratory on weekends or holidays. TEM vials must be received in the diagnostic laboratory within 48 hours after the vials are inoculated. Samples that reach the laboratory after this time period will be considered unfit for analysis.
- Samples must be protected from extremes of temperature during transit. Any factor that affects the viability of the agent in question will affect the sensitivity of the test and therefore the reliability of the test result. If samples arrive at the laboratory cold or frozen, they will not be analyzed and the result will be reported as unfit.
Special consideration
13. For security reasons, in the case of an intractable animal where it is not possible to take a second sample when testing for both organisms, the single sample taken may be processed as follows. Please note however that following this procedure may negatively impact the sensitivity of the respective tests. The first half of the sample is inoculated into the TFTM media from the Saskatoon laboratory by expressing sample material onto the inside wall of the tube directly above the TFTM media. The tube should be re-capped and inverted several times to mix the sample into the media. The second half of the sample is inoculated in the saline vial provided by the Ottawa laboratory for Campylobacter testing. For further processing of each sample, refer to the above-mentioned procedures (11 and 12 above). Do not transfer the entire sample into the saline vial, and then split it between the TFTM and TEM media. The sample for Tritrichomonas must not have been diluted in saline before inoculation in the TFTM media, as this can further reduce the sensitivity of the test.
Note: This procedure must be reserved for exceptional cases only where the collection of two separate samples would be unsafe for the collector.
Submission forms
14. Submission forms are available at the CFIA district office. A copy of the appropriate completed submission form must be sent to the district office, and for each test requested, a copy of the submission form must be sent to the laboratory along with the samples.
15. Care must be taken when filling out the forms. All copies must be legible. All information should be recorded on the form, including ear tag numbers, tattoos or other types of identification. The type of tests being requested, and the date and location where the specimens were collected, should be recorded. Accredited veterinarians must include their name, address (including postal code) and telephone number, and must sign at the bottom of the form. The submitter code must also be indicated, as well as the identification code of the CFIA district office in which the animals are located.
16. CFIA/ACIA Form 5473 – Animal Health Import, Export and Artificial Insemination Specimen Submission must be used. Instructions for submission of samples are printed on that form. A laboratory notification number, which is available from a CFIA district veterinarian, must be entered in the "Reason for Test" section of the form. The CFIA laboratory will not perform the analysis if the notification number is missing.
17. It is the responsibility of the accredited veterinarian to provide all information required, or to resubmit if a specimen is unfit for testing. Laboratories may refuse to conduct analysis of samples submitted with forms that are not fully completed. Refer to a CFIA district veterinarian for examples of completed forms, and for instructions on distribution of forms for samples being sent to CFIA laboratories.
Shipping samples
18. It is essential that samples be shipped in a timely fashion to the appropriate laboratory to avoid unnecessary re-sampling. If uncertain, check with a CFIA district veterinarian before shipping.
19. Vial breakage can result in re-sampling an animal and significantly prolong laboratory test result turnaround times. In addition to a legal responsibility, a submitter has a moral obligation to ensure the safety of transportation industry workers and laboratory staff handling the specimen. The use of an appropriate container is a requirement when submitting samples to the laboratory.
20. Accredited veterinarians must make sure that the packaging and shipping procedure used complies with regulations concerning the transportation of such samples.
21. The laboratory submission forms should be placed in an envelope and attached to the outside of the protective containers.
22. The shipment of samples must never be entrusted to the animal owner or exporter. The accredited veterinarian must be able to maintain a chain of custody for samples shipped to the laboratories.
23. The accredited veterinarian is responsible for shipping costs.
Laboratories
24. Samples for Campylobacter testing must be sent to the CFIA Ottawa laboratory (Fallowfield). Samples for Tritrichomonas testing must be sent to the CFIA Saskatoon Laboratory. CFIA laboratory information may be found in module 3.3.
Test results
25. Test results reported from CFIA laboratories are generated through the Laboratory Sample Tracking System (LSTS). The test results will be provided to the accredited veterinarian by the local district office. The accredited veterinarian is responsible for advising the owner of the results.
Laboratory fees
26. The accredited veterinarian is responsible for laboratory fees. The district office will recover applicable fees for all animals that had tests performed in a CFIA laboratory, regardless of the test result. The Canadian Food Inspection Agency Fees Notice can be consulted for more information.
3.5 Genotype Testing for Scrapie Susceptibility
Sheep
Genotype analysis of the prion gene has been determined to be a significant factor in susceptibility to infection with classical scrapie in sheep. One is most likely to discover the disease in sheep in genotypes with the highest susceptibility (See Table 1 below).
The genetic makeup of sheep, as with all mammals, are diploid organisms. All cells contain two copies of each chromosome, and thus, two copies of the gene that codes for the prion protein. Genes are made up of codons, a stretch of DNA that determines which particular amino acid will be included at a particular location of a protein (in this case, the prion protein). The prion protein is comprised of 256 amino acids, so there are 256 codons determining these amino acids in the prion protein gene (PRNP).
Studies from various geographic areas and different breeds of sheep have found three codons to be most significantly related to susceptibility to classical scrapie: 136, 154, and 171. The genotypes of sheep in Canada are referred to using these codon number and the corresponding two alleles associated with the codon. For example, 136 AV; 154 RR; 171 QR (also referred to as ARQ/VRR in short hand). In North America, codons 136 and 171 are given primary importance in association with susceptibility to classical scrapie.
The profile of the sheep's prion genotype varies among countries, flocks, and breeds. While there is no genotype that confers absolute resistance to scrapie infection, the presence of arginine (R) at codon 171 confers a high level of resistance to classical scrapie. Conversely, the presence of glutamine (Q) at codon 171 is associated with susceptibility. This is supported by the fact that of all sheep diagnosed with classical scrapie in Canada from 1998–2008, 93% were of the genotype ARQ/ARQ, and over 98% were homozygous 171QQ. Similarly, the majority of classical scrapie cases reported worldwide have been in 171QQ sheep.
Although rare in the Canadian sheep population, histidine (H) or lysine (K) may also be found at codon 171, but both are currently considered equivalent to 171Q in term of susceptibility. The valine (V) amino acid at codon 136, confers greater susceptibility among the 171QR population. Table 1 summarizes the relative susceptibility of sheep genotypes to classical scrapie.
| Genotype (136, 171) | Susceptibility to classical scrapie |
|---|---|
| AA, RR | Negligible |
| AA, QR | very low |
| AV, QR | intermediate |
| AA, QQ AV, QQ VV, QQ |
high |
A significant finding, as it relates to the transmission of scrapie, is that the genotype of the fetus influences the migration and accumulation of abnormal prion in the placenta of an infected ewe. A 171QQ-infected ewe carrying a 171QQ fetus results in the accumulation of large quantities of abnormal prion, which is subsequently shed during birth or abortion. However, when a scrapie-infected ewe carries a 171QR or 171RR fetus, the abnormal prion does not cross to the fetal side of the placenta. The use of a 171RR ram for breeding, therefore, can prevent the shedding of abnormal prion at lambing from infected ewes.
The knowledge of genetic resistance to classical scrapie has led to the implementation of breeding programs in several countries that are aimed at reducing national flock susceptibility. Although the heavy promotion of the use of 171RR rams across Canada may be effective in minimizing the spread of scrapie, it would also result in a significant change in the genotype profile of the national flock. Currently, Canada's sheep population demographics show considerable variation in scrapie genotype profiles between breeds and flocks.
Breeding for scrapie resistance is an effective disease control tool that may be applied by owners. To date, the selection for resistant scrapie genotypes has not been associated with negative production traits.
Note that atypical scrapie does not follow the same association with genotype as described above for classical scrapie and has been detected in numerous countries, primarily through the testing of deadstock and active scrapie surveillance programs. Although now referred to as atypical scrapie, cases were initially referred to as Nor98, as the first case was identified in Norway in 1998. Atypical scrapie appears to be clinically, pathologically, biochemically, and epidemiologically unrelated to classical scrapie and therefore not a known risk for spread as it is limited to that individual animal.
Goats
Over the last several years, CFIA evaluated the evidence for genetic resistance to classical scrapie in goats. Genotype data obtained from several scrapie-infected goat herds in Canada, combined with ongoing research findings from North American and European studies, provide convincing evidence for genetic resistance in goats.
The CFIA is currently work towards the incorporation of the use of genetic resistance alleles S146 and K222 in goats in our Canada's domestic National Scrapie Eradication Program (NSEP). This is consistent with our trading partners, the United States (US) and the European Union (EU). Internationally, other scrapie-affected countries (including the US) with eradication programs have started to use in their domestic programs.
Further research in the Canadian context is still needed for goat genotypes and resistance to scrapie, and will be done via a pilot project to assess for scrapie resistance in positive goat herds. and The hope is that goat genotyping can be fully incorporated into the NSEP in the future, along with the goal of ultimately being able to also use goat genotyping for scrapie resistance for import and export purposes.
Sampling procedures for genotyping
Samples taken for genotyping for the purposes of the National Scrapie Flock Certification Program (SFCP) or for export the U.S. must be taken by an accredited veterinarian. Approved identifiers are required on sheep being genotyped and the official identification number (15 digits) included with the sample submission.
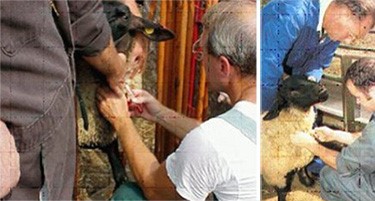
Jugular venipuncture
To facilitate the rapid collection of blood samples from multiple sheep in as little time as possible, it is suggested to not waste time upending the sheep. Restrain sheep for venipuncture in a standing position.
Occlude thoracic inlet, palpate or approximate the location of the jugular vein, and insert Vacutainer needle.
A single-use needle is required for each animal, to prevent cross-contamination of DNA.
Collect a full 7-10 ml of blood in an ethylenediaminetetraacetate (EDTA) tube (lilac or purple top).
Make sure that the sample and individual animal identification are irrefutably connected.
Samples should be stored at 4°C until shipped. Ship to laboratory as soon as possible.
Module 3.2 of this manual proves detailed instructions on the packaging, shipping and submission of serological samples.
Samples collected under the Scrapie Flock Certification Program should be submitted to a lab listed in Annex G of Module 7.5 of this manual.
Samples collected in support of export to the US, should be submitted to the National Veterinary Services Laboratory (NVSL) or another laboratory designated by NVSL to verify genotype. Select "Ovine PrP Genotyping" at the link below to access lab information.
Alternate tissues samples
Genotype analysis may be performed on tissues other than whole blood provided that the approved lab has been evaluated and approved for testing of the type of tissue submitted (for example, skin biopsy). It is the responsibility of the accredited veterinarian to confirm what tissue testing is offered under the approved lab's scope of duties.
- Date modified: