Chapter 6 – Export to Mexico
This page is part of the Guidance Document Repository (GDR).
Looking for related documents?
Search for related documents in the Guidance Document Repository
Table of Contents
- 6.1 General requirements
- 6.2 Horses (updated December 2019)
- 6.3 Swine (updated July 2018)
- 6.4 Cattle (updated December 2017)
- 6.5 Sheep and goats (updated June 2016)
- 6.6 Cervids (updated April 2018)
6.1 General requirements
General requirements
Livestock, semen, and embryos imported into Mexico require a "hoja de requisitos zoosanitarios" (HRZ) or list of requirements and statements that Canada's veterinary health certificate must include. This condition is similar to an import permit and, on occasion, the requirements change without notification to Canada. Exporters should verify that the requirement for an importer to obtain the "hoja de requisitos" has been met and that the conditions listed in that document are satisfied by Canada's export certificate.
Shipments of swine, sheep and goats must follow the United States Department of Agriculture Animal and Plant Health Inspection Services (USDA APHIS) "Protocol for the transit of swine, sheep and goats from Canada to Mexico by land through the United States".
Trucks transporting swine or horses for export from Canada to Mexico through the U.S. usually will be sealed at the U.S. port of entry, and the seals will be removed at the port of entry into Mexico. However, trucks transporting cattle must be sealed with official Canadian Food Inspection Agency (CFIA) seals at the farm of origin in Canada. The official seals must be applied by an accredited veterinarian or a technician designated as an inspector pursuant to the Canadian Food Inspection Agency Act, for the limited purposes of the Health of Animals Act. This designation allows only the accredited veterinarian or their technician to affix the seals. Removal of these official seals must be pre-authorized by a CFIA inspector.
All inspections must be made by an accredited veterinarian authorized by the Accredited Veterinarian Agreement, and every animal must meet each of the conditions required for their export to Mexico.
It is up to the exporter or Mexican importer to apply for an in-transit permit from the United States Department of Agriculture (USDA) and arrange for feed and rest stations on trips of long duration. Livestock inspected and certified for export to Mexico are not required to be certified for export to the US They are considered to be "in transit" only, and can cross the US with their Mexican certificate and an in-transit permit from the USDA. However, if the animals are not certified for export to the US and a problem arises at the Mexican border, they would not be allowed to remain in the US except for the purpose of immediate slaughter. In this situation, the animals could also be returned to Canada. Animals under permit transiting the US should not travel with animals legally entering the U.S. on the same transport vehicle.
Note: in such cases where entry to Mexico is denied and animals are required to return to Canada, exporters must be aware of the transportation of animals requirements regarding time in transport and feed / water / rest intervals. It may be necessary to arrange additional feed and rest periods in order to comply with these requirements while returning to Canada. If return to Canada would not be a desired option, exporters may choose to have their animals certified for export to the U.S. (using the appropriate veterinary health certificate) as well, which would allow them to stay at a U.S. destination pending resolution of the issues denying entry to Mexico.
In completing the health certificate to export swine to Mexico, "port of departure" refers to the Canadian port from which animals leave Canada. "Destination" is the Mexican destination provided by the Canadian exporter.
Mexican authorities do not accept hand written certificates. The certificate must be typed, including the reference number. Fillable PDF certificates to Mexico are available from the CFIA district office. Please contact your district office for more information.
The export certification is considered complete and valid only when it has been endorsed and stamped with the official export stamp by a CFIA veterinary inspector. The veterinary inspector is usually the district veterinarian responsible for the area in which the herd of origin is located or another veterinary inspector if prior arrangements have been made.
The period of time that an export certificate remains valid is based not only on the date that the completed certificate is endorsed, but also on the actual date that the inspections, treatments or tests were performed.
6.2 Horses (updated December 2019)
Health certification
1. The veterinary health certificate is the HA1263 Export of horses to Mexico / Exportación de caballos a México.
2. The status of the HA1263 certificate must be verified with the local CFIA district office before beginning testing to ensure that the certificate is current and matches the "hoja de requisitos" and that Canada is free of diseases mentioned in article 1 of the export certificate.
3. Animals must be individually identified with a microchip with the number included on the health certificate.
The animals need to have an additional proof of identification (for example: the FEI passport or the Identification addendum for horses exported from Canada to Mexico) that clearly and uniquely identifies the animals and includes verifiable visual characteristics. The microchip number must also be included on this identification document. A pdf fillable copy of the addendum is available at the CFIA district office, if needed.
4. The horses must have been in Canada for 60 days prior to shipping. The animals must be officially isolated for three weeks prior to exportation. The accredited veterinarian must approve an isolation area within the facility. The isolation area must prevent any nose-to-nose contact with other animals not intended for export to Mexico. In order to be recognized as an "official" isolation, the accredited veterinarian must declare to the CFIA district office all the information related to the isolation facility (name and address of the facility, description of the isolation area, contact name and telephone number of the stable manager, start date of isolation and intended export date), within 5 days after the beginning of the isolation period. CFIA employees may visit the facility at their discretion.
Note: The 60-day residency period and 3 week isolation requirements do not apply to sport horses over 4 years old dedicated to jumping, dressage and full events, exported temporarily to Mexico. Those horses must have remained in North America (Canada, United States and Mexico) in the last 3 weeks under veterinary supervision, and have not had signs compatible with infectious or contagious diseases.
5. The premises of origin have not been in quarantine, and have not had reported cases of dourine, glanders, surra, equine coital exanthema, Venezuelan equine encephalitis, epizootic lymphangitis, ulcerative lymphangitis, and infection by Salmonella abortus equi.
6. The horses were not vaccinated with live or attenuated vaccines during the thirty (30) days prior to exportation.
7. Vehicles used to transport the horses must be cleaned and disinfected before loading, and can only carry horses qualified for export to Mexico during the trip.
Certification procedure
8. The animals must be inspected prior to exportation and found healthy without any evidence of infectious or contagious diseases and free of ectoparasites or fresh wounds.
9. The animals must be tested for equine infectious anemia using the agar gel immunodiffusion (AGID) test or the ELISA test with negative results within six (6) months before exportation. An EIA test certificate that has an inaccurate description or drawing of the horse cannot be used to support certification for export. An accredited veterinarian is not authorized to modify any information written on an EIA test certificate once test results have been recorded. In order to certify a horse in this situation, the accredited veterinarian may, at the owner’s request, re-sample the horse for testing and wait for the new result before export certification may be completed.
10. The animals must be either tested or vaccinated for the following diseases: equine viral arteritis, eastern and western encephalitis (EEE and WEE) and equine rhinopneumonitis. It is highly recommended that vaccinations be chosen. All vaccinations using live or attenuated vaccines must have been given more than 30 days prior to exportation. If the animals have to be tested for one of these diseases, the test must be performed within 60 days of exportation. The accredited veterinarian must contact the district office to obtain instructions about the testing procedure. Samples for equine viral arteritis and equine encephalitis may be analyzed in CFIA laboratories. In these cases, the instructions provided in section 3.2 of the manual "Serologic Testing" must be followed and a specific notification number must be obtained from the district office. CFIA laboratories do not perform tests for equine rhinopneumonitis. If a test is required, a private laboratory that meets the requirement of the Policy on the Use of External Laboratories for Export Testing must be selected. CFIA district office must be contacted to obtain more information about this policy before samples are sent to a private laboratory.
11. Animals must be vaccinated against influenza and the vaccination must be valid. No vaccination with a live or attenuated vaccine is permitted within 30 days of export.
How to complete the Canadian health certificate (HA1263)
12. The accredited veterinarian must use the most recent version of the HA1263.
13. Mexican authorities do not accept hand written certificates. The certificate must be typed. A fillable PDF certificate is available through the district office. The reference number should be requested in advance from the district office, to avoid hand writing it on the certificate.
14. Vaccinations and tests must be reported in Table 1. In the case of vaccination, the name of the vaccine, lot number and date of vaccination are required. For eastern and western equine encephalitis, the last two vaccination dates are required. In the case of tests, the test type and negative test result "N" must be indicated.
15. The accredited veterinarian must complete the export health certificate in English by entering all required information according to the directions provided above. The "Reference number" is provided by the CFIA district office. The completed health certificate along with a copy of the EIA test result shall be submitted to a CFIA veterinary inspector to review and, if all the requirements have been met, the certificate will be endorsed. An incomplete export certificate will be returned to the accredited veterinarian to be completed. A fee will be charged for the CFIA's endorsement. Endorsed certificates will be returned to the accredited veterinarian. The health certificate is valid for a period of 30 days from the date of inspection recorded on the certificate.
6.2A Re-entry to Mexico
- The veterinary health certificate is the HA2883 Re-export of horses to Mexico. This certificate is valid for the return to Mexico, within 60 days of the horses being imported into Canada.
- There are no test requirements.
- Canada must be free of contagious equine metritis and foot-and-mouth disease.
- At inspection prior to return, the animals were found clinically healthy and free of ectoparasites and without any open wounds; animals with myiasis or fresh wounds are not accepted. The horses haven't presented any signs of infectious or transmissible disease during their stay and have been isolated from horses with an inferior health status.
- The horses did not have reproductive activity during their stay in Canada.
- The horses were not in premises where Taylorella equigenitalis was detected, nor in premises under quarantine, nor under investigation for Contagious Equine Metritis (CEM).
- Mexican authorities do not accept hand written certificates. The certificate must be typed. A fillable PDF certificate is available through the district office. The "Reference number" should be requested in advance from the district office, to avoid hand writing it on the certificate.
- The accredited veterinarian must complete the export health certificate in English by entering all required information. The completed health certificate shall be submitted to a CFIA veterinary inspector to review and, if all the requirements have been met, the certificate will be endorsed. An incomplete export certificate will be returned to the accredited veterinarian to be completed. A fee will be charged for the CFIA's endorsement. Endorsed certificates will be returned to the accredited veterinarian.
References
Copies of certificates HA1263 and HA2883 are available at the CFIA district office.
6.3 Swine (updated July 2018)
Authorization
Veterinarians authorized to certify swine for export to Mexico may certify both breeding swine and wild boar.
Breeding swine – Health certification
1. General requirements described in section 6.1 must be reviewed.
2. Certificate HA1240 Export Swine to Mexico / Exportación de cerdos a México must be used.
3. The animals must meet all the export certificate requirements.
4. Canada must be free from brucellosis, classical swine fever and pseudorabies (Aujeszky's disease).
5. Mexican authorities require each animal to be identified with a unique identification number. The animals may be identified with:
- ear tags that bear a unique 15 digit number that follows the ISO 11784 standard format. These tags can be either electronic or non-electronic; or
- ear tags which bear an official 5 character alphanumeric CPC-designated herd mark unique to the production site, with a secondary unique herd management identification number on the same tag; or
- registered individual ear tattoos that permit trace back to the herd of origin.
6. The animals being exported must be inspected by an accredited veterinarian. Swine must be found to be free of clinical signs of infectious, contagious, or parasitic disease.
7. The animals did not present with clinical signs of disease within thirty (30) days prior to shipment, including influenza, based on veterinary inspection.
8. The animals being exported must be clinically free of atrophic rhinitis and originate from farms on which there have been no reported cases of atrophic rhinitis for at least 12 months before the date of exportation.
9. The animals being exported must originate from farms on which clinical cases of porcine reproductive and respiratory syndrome (PRRS) have not been present during the previous three months and which have not introduced swine from farms on which PRRS has been identified during the previous 30 days.
10. The animals exported to Mexico came from farms that did not have any cases of porcine epidemic diarrhea within the last 6 months.
11. The animals being exported must originate from herds where transmissible gastroenteritis has not been diagnosed during the past six months.
12. The animals being exported must have been vaccinated against the porcine circovirus type 2, prior to shipment.
13. The animals must be transported in cleaned and disinfected vehicles and must not be exposed to other livestock during transportation.
Wild boar to Mexico – Health certification
14. General requirements described in section 6.1 must be reviewed. The exporter is required to send a request to Mexico in advance for every shipment. Contact the animal health district office for more information regarding SENASICA contact and required information that must be provided.
15. The last version of certificate HA2993 Export of wild boar to Mexico/Exportación de jabalies a México must be used. The terms and conditions of export are subject to change from time to time and without notice. The status of the export certificate (HA2993) must be verified with the local CFIA district office prior to the commencement of testing, to ensure it is current and matches the list of conditions contained in the "hoja de requisitos" (document similar to an import permit).
16. Canada must be free from foot and mouth disease, classical swine fever, African swine fever, swine vesicular disease, Aujeszky's disease (pseudorabies) and brucellosis in swine (B. suis, B. abortus).
17. The wild swine for export were born in Canada and have been on the premises of origin for at least three 3 months.
18. Transmissible gastroenteritis (TGE) has not occurred on the premises of origin during the 6 months prior to export
19. Mexican authorities require each animal to be identified with a unique identification number. The animals may be identified with:
- ear tags that bear a unique 15 digit number that follows the ISO 11784 standard format. These tags can be either electronic or non-electronic; or
- ear tags which bear an official 5 digit alphanumeric CPC-designated herd mark unique to the production site, with a secondary unique herd management identification number on the same tag; or
- registered individual ear tattoos that permit trace back to the herd of origin.
20. The swine were not vaccinated during the 14 days preceding export.
21. The swine were inspected within 30 days of the date of export and found to be free of evidence of infectious and communicable disease, ectoparasites and fresh unhealed wounds. At the time of inspection, the swine did not present with clinical signs of atrophic rhinitis. The date of inspection must be recorded.
23. The truck used for transport was washed and disinfected prior to loading the swine intended for export to Mexico, with a disinfectant authorized by the Government Canada.
Wild boar to Mexico – Certification procedure
24. Animals to be exported must be tested with negative results within 30 days of export for the following diseases:
- Brucellosis: The tests required on the health certificates (FPA or ELISA) are not performed in CFIA approved laboratories. The samples must be sent into CFIA laboratories, Lethbridge or Ottawa. The FPA must be selected.
- Aujezsky's disease: The sample must be sent to the CFIA Winnipeg laboratory and the ELISA test must be selected.
Note: In order to submit samples to a CFIA laboratory, use Form CFIA/ACIA 5473 – Animal Health Import, Export and Artificial Insemination Specimen Submission. An export notification number must be written on the submission form. This notification number should be requested from the CFIA district office. The electronic submission of a template for sample information is mandatory. Consult Module 3.2 Serologic Testing for more information regarding the special procedure for submission to CFIA laboratories, applicable to samples from bovine tested in isolation (IAI notification), and from sheep and goats, wild swine or cervids for export to Mexico.
25. The swine have been subjected to a preventive treatment against internal and external parasites within 30 days prior to their export.
How to complete the Canadian health certificates (HA1240, HA2993)
26. The accredited veterinarian must use the most recent version of the export certificate. The accredited veterinarian who inspected the animals must sign the health certificate.
27. Mexican authorities do not accept hand written certificates. The certificate must be typed, including the "Reference number". The Reference number is assigned by the CFIA district office. The export certificate must not contain cross-outs, changes or errors. Fillable PDF certificates to Mexico are available from the CFIA district office.
28. The identification of each animal must be reported on the certificate including the identification number and a description of the animal.
29. The completed certificate will be submitted to a CFIA veterinary inspector to review and, if all requirements are met, it will be endorsed. Incomplete export certificates will be returned to the accredited veterinarian for completion. A fee will be charged for CFIA endorsement. Endorsed certificates will be returned to the accredited veterinarian. The health certificate HA1240 for breeding swine is valid for 30 days from the date of inspection. The health certificate HA2993 for wild swine is valid for 30 days from the inspection, treatment or test date.
References
The export health certificates HA1240 and HA2993 are available at the district office.
6.4 Cattle (updated December 2017)
This section describes inspection and certification requirements for the export of cattle to Mexico.
Authorization
Veterinarians accredited for this function are authorized to certify cattle for export to Mexico. Currently, there is a certificate for breeding cattle (HA1296).
Veterinarians accredited for this function are required to maintain records to document how certification elements have been met for each shipment.
General certification requirements
1. General information about exportation to Mexico mentioned in module 6.1 must have been reviewed. The general principles that apply to inspecting and testing cattle for export to the U.S. also apply to inspecting and testing cattle for export to Mexico.
2. The accredited veterinarian must use the most recent version of the export certificate. The terms and conditions of export are subject to change from time to time and without notice. The status of the Canadian Food Inspection Agency (CFIA) export certificate (HA1296) must be verified with the local district office of the CFIA prior to the commencement of testing, to ensure it is current and matches the list of conditions contained in the "hoja de requisitos" (document similar to an import permit).
3. The animals must be individually identified with tags approved under the Livestock Identification and Traceability (TRACE) Program. Approved tags for cattle bear a unique identification number. The identification numbers of approved tags start with "124 000 0", "124 000 1" or "124 000 2". Tags considered equivalent under the TRACE Program that start with "840" are also acceptable as official identification.
Note:
- Even though it is not a requirement of the importing country that the ear tag numbers be listed in ascending numerical order on the certificate, accredited veterinarians are encouraged to complete certificates in this manner. This practice will facilitate inspection at the ports of entry and minimize delays.
- The animals must not bear a light blue, Allflex, tamperproof, dangle tag in their left ear, marked with CFIA/ACIA and a 4-digit number, as these animals cannot be certified for export to any country.

4. In the column "Age", record the age in months. The only official requirement is that the exported animals must have been born on or after January 1, 1999; however, it is important for the exporter and the accredited veterinarian to know that Mexican importers may receive subsidies from the Government of Mexico if they can prove that the imported animals are less than 30 months of age.
Non-specific age entries are not acceptable, e.g. <30 months, but an approximation based on dentition is acceptable (see section 23 below).
5. The breed and sex must be recorded in full on the certificate. Abbreviations are not allowed. In the case of registered cattle, the registration number must be entered in the last column (description).
6. The Mexican authorities do not accept hand written certificates. The certificate must be typed, including the "Reference number". The reference number should be requested in advance from the district office, to avoid hand writing it on the certificate. A fillable PDF certificate is available from the district office.
7. The accredited veterinarian must complete the export health certificate by entering all the necessary information with the exception of the number of animals in the shipment and the seal numbers. The completed and signed health certificate shall be submitted to a CFIA veterinary inspector to review and, if all requirements have been met, the certificate will be endorsed. An incomplete export certificate will be returned to the accredited veterinarian for completion. A fee will be charged for CFIA endorsement. The endorsed certificate will be returned to the accredited veterinarian.
8. The original and three copies of the official export certificate must accompany the shipment.
9. The animals are loaded at the farm of origin under the supervision of the accredited veterinarian or his designated technician, and the trucks are sealed.
10. An addendum (see "Addendum for Rest Stops" in section 13) will be added to the certificate for transit in the U.S. by United States Department of Agriculture (USDA) veterinarians or USDA accredited veterinarians in order to record when the seals are broken and re-applied.
11. If the seals are broken or missing, or if they do not match the seal numbers recorded on the health certificate or the USDA's addendum, the shipment will be refused entry into the U.S. or Mexico.
12. From the farm of origin to the U.S. border, the seals cannot be broken by anyone other than a CFIA inspector or a person under the CFIA inspector's supervision. If the exporter needs to transfer animals from one truck to another after leaving the farm of origin, the transfer must be supervised by either a CFIA inspector, or an accredited veterinarian or technician designated to apply seals. The accredited veterinarian or technician must first obtain authorization from a CFIA inspector to remove seals for that specific situation. More information can be found in the section Use and Control of CFIA Seals. After changing the seals, the responsible person will issue an official letter to confirm the change in the seal numbers. If a CFIA inspector was required to provide this service, a fee will be charged.
During transit in the U.S., the USDA should always be contacted before breaking any seal on the truck.
USDA protocol to transit ruminants from Canada to Mexico
13. A Canadian health certificate to export cattle to Mexico (HA1296) and a USDA transit import permit will be required to transit in the USA. Exporters transporting loads of cattle through the USA to Mexico are required to observe the USDA Protocol to Transit Canadian bovines to Mexico. The following documents are available on the USDA Website:
- Questions and Answers Transit Canadian Bovines to Mexico
- Transit Canadian Bovines to Mexico
- Addendum for Rest Stops
In case of refused entry in Mexico
14. The exporter needs – and should be advised by the accredited veterinarian – to have contingency plans in place for the handling of the shipment should it be refused entry in Mexico. For shipments which are refused entry, there are three possible outcomes:
- they return to Canada (please check with the CFIA for requirements to return these animals);
- they are sent to a U.S. slaughterhouse under a special permit from the USDA; or,
- the animals can be sold within the U.S. if they have been inspected and certified to meet U.S. requirements. In this case, the accredited veterinarian must also be accredited for the export of cattle to the U.S. and the endorsed certificate HA1941 must have accompanied the load into the U.S. Since the certificate for the U.S. will be used only if the shipment is refused entry in Mexico, it does not need to be shown on entry in the U.S. If needed, the inspection will be done by a USDA veterinarian at the Mexico-U.S. border's point of inspection, and the decision to let the animals enter the U.S. will be taken at this moment.
If the CFIA district office is asked to certify a shipment for both countries, the usual certification fees will be charged for both certificates.
Use and control of CFIA seals
15. The export of cattle to Mexico must be done in vehicles that are sealed at the location from which the animals are being shipped. Seals must be applied by the accredited veterinarian who signs the certificate or by a technician designated by the accredited veterinarian.
16. To perform this function, accredited veterinarians or their technicians must be designated under the Health of Animals Act to affix official seals. Contact the CFIA district veterinarian to obtain a designation certificate. Please note that this designation does not allow the removal of CFIA official seals. For each situation where official seals must be removed, the designated accredited veterinarian or technician must contact their CFIA district office in advance to obtain an authorization to remove official seals.
17. Accredited veterinarians will provide the CFIA district veterinarian with the names of any technicians able to perform the duties of sealing vehicles for the shipment of animals to Mexico. This list must be updated as soon as changes in staff are made.
18. The CFIA's Animal Health district office will provide seals for the vehicles. Seals may be allocated to an accredited veterinarian or to a veterinary clinic when more than one accredited veterinarian is employed by the same clinic.
19. The district office will keep records containing the seal numbers and the names of the accredited veterinarians or veterinary clinics to which the seals were distributed.
20. Once seals are applied to all possible exits of a vehicle transporting livestock, accredited veterinarians or their designated technicians must record the numbers on the official export certificate in the appropriate section and initial the appropriate section.
21. Accredited veterinarians are responsible to keep records of the seals that were used. Seal numbers must be matched with export certificate numbers. These records must be kept for a minimum of three years.
22. Accredited veterinarians must submit, upon request, a list of the seals used and the corresponding export certificate numbers to the CFIA's Animal Health district office. The following list can be put in a table and used to submit this information. The document can be sent by facsimile, electronic mail or regular mail.
- Accredited Veterinarian or Technician
- Seal Numbers
- Export Certificate Reference Number
- Date of Application of Seals
- Truck or Trailer License Plate Number
Age determination
23. The following will apply to age determination:
- Dentition: animals are eligible to be exported to Mexico after a visual inspection of dentition, provided the eighth permanent incisor has not erupted. The definition of eruption is the emergence/penetration of the tooth/teeth through the gingiva (gum line). If the eighth incisor has erupted, the age of the cattle will need to be verified by a birth record (see definition in article ii below). For additional information, please visit the following Website: USDA, Food Safety and Inspection Service (FSIS) – Using Dentition to Age Cattle
Click on image for larger view
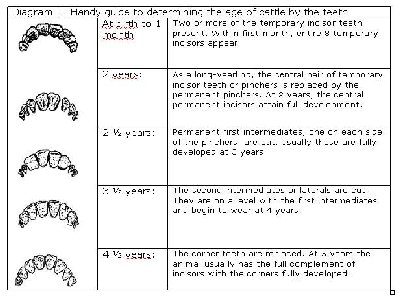
Description for picture – Dentition
Diagram – Handy guide to determining the age of cattle by the teeth:
At birth to 1 month: Two or more of the temporary incisor teeth present. Within first month, entire 8 temporary incisors appear.
2 years: As a long-yearling, the central pair of temporary incisor teeth or pinchers is replaced by the permanent pinchers. At 2 years, the central permanent incisors attain full development.
2 ½ years: Permanent first intermediates, one on each side of the pinchers, are cut. Usually these are fully developed at 3 years.
3 ½ years: The second intermediates or laterals are cut. They are on a level with the first intermediates and begin to wear at 4 years.
4 ½ years: The corner teeth are replaced. At 5 years the animal usually has the full complement of incisors with the corners fully developed.
- Birth record: Agri-traçabilité Québec (ATQ) database, purebred record and registration certificate, Canadian Cattle Identification Agency (CCIA) database, or other record (including birth farm records) that provides evidence acceptable to the accredited veterinarian indicating the animal was born on or after January 1, 1999. If animals being certified fall into this category, the accredited veterinarian must validate the information and maintain records of the information source with each shipment that is certified for export. The word of an exporter by itself is insufficient to satisfy the requirement of a birth record. Information about birth records and measures employed to validate those records should be made available to a CFIA veterinarian upon request.
- Visual inspection: visual age verification is allowable for certification of younger animals which may be presented to an accredited veterinarian. Visual inspection may be used for animals which are under 3 years of age. It is recognized that the experienced practitioner is able to estimate the age of these young animals upon visual inspection, and that the accredited veterinarian is often knowledgeable about the cattle to be exported (for example, feed lots where cattle are essentially penned according to birth year or veal operations). Visual verification should only be used when the accredited veterinarian is confident about the age of the animals. If there is any concern that the animal(s) being inspected does not fall into this category, the accredited veterinarian should choose option i) or ii) above for age verification. As the animals get closer to an estimated age of 3 years, the accredited veterinarian should have more knowledge regarding the history of the animals, especially if the animals have been just recently assembled.
HA1296 – Breeding cattle to Mexico: requirements and completion
24. The cattle certified for export are either born and raised in Canada or legally imported from the U.S.
25. The cattle certified for export were born on or after January 1, 1999.
26. The exported cattle are identified with permanent identification approved under the Health of Animals Regulations, which allows the tracking of the animals to the herd of origin.
27. Canada is classified by the World Organisation for Animal Health (WOAH; founded as Office International des Épizooties (OIE)) as a country with controlled risk with respect to BSE and complies with the conditions described in article 2.3.13 of the Terrestrial Animal Health Code (2007).
28. During the inspection prior to exportation, the animals were found to be clinically healthy.
29. Bovine brucellosis
The animals to be exported originate from herds officially free of brucellosis and they were submitted to an individual serological test with negative results (in accordance with Canadian Standards) within 60 days prior to the date of export. If the animals are less than six (6) months of age on the day of the shipment, the test is not required. The sample for brucellosis testing is submitted to a private laboratory approved by CFIA.
The date of sampling must be recorded in section 6. In the case where sampling has been done on different days, record the earliest date.
30. Bovine tuberculosis
All animals over 1 month of age at the time of export must be tested and found negative to the caudal fold intradermal tuberculin test, using 0.1 ml of PPD tuberculin. The test must be completed within 60 days of export.
The date of reading of the tests must be recorded in section 7. In the case where testing has been done on different days, record the earliest reading date.
31. The animals must be free of campylobacteriosis (C. fetus ssp. venerealis) and trichomoniasis. The following animals are exempted from testing:
- bulls that are issued from embryo transfer or artificial insemination, and have never been used for natural mating other than with virgin heifers
- heifers that have never mated naturally or that have been bred with artificial insemination only, or that have been bred with bulls exempted from testing as described in i) above.
If these conditions cannot be met, then animals must be tested with negative results.
Testing can be done either by CFIA staff or accredited veterinarians authorized to perform this function. Information on the procedure may be found in module 3.4
32. Canada is free of piroplasmosis.
33. The animals were inspected and found to be free of clinical signs for infectious bovine rhinotracheitis and leptospirosis. They were vaccinated against infectious bovine rhinotracheitis and leptospirosis, using a bacterin against five strains of Leptospira (L. pomona, L. icterohaemorrhagiae, L. canicola, L. grippotyphosa and L. hardjo). For both diseases, the vaccine was administered between 10 and 90 days prior to the date of export.
34. The animals are free of ectoparasites.
35. Canada is free of Boophilus spp. ticks.
36. The animals in the shipment were inspected by an official veterinarian or a veterinarian accredited by the CFIA, and were loaded under his/her supervision. The loading may be performed under the supervision of a designated technician.
37. From the point of shipping until their departure from Canada, the animals will be transported in sealed vehicles (see "Use and Control of CFIA Seals".
38. The vehicles used for the transportation of the animals were cleaned and disinfected prior to the loading of the animals.
39. A small number of "reserve" animals can be recorded on the export certificate. No strikeouts are allowed on the export certificate for the animals that are not loaded on the truck at the moment of departure.
40. A list of animals may be attached to the export certificate as long as it contains the same information as the table in the Appendix I. In this case, it must be recorded in the table of Appendix I that the list of animals included in the shipment is attached. This list must be initialed by the accredited veterinarian and "CFIA" must be stamped on every page. The reference number must also be recorded on each page and pagination must be present. The rest of Appendix I must be completed as usual.
41. The accredited veterinarian or their technician must, on the day of export, return to the farm, supervise the loading and apply CFIA seals to the transporting vehicles, after verifying that only the animals listed on the export certificate are included in the shipment. A CFIA seal must be applied to every door on the trailer.
42. The accredited veterinarian or their technician must record the number of animals in the shipment and the CFIA seal numbers in the appropriate section on the endorsed original health certificate, and initial the appropriate section. Please repeat this information on the copies of the certificate, as well as on the copy kept in the accredited veterinarian file.
6.4A Export to Mexico: certification requirements of cattle: multiple farms of origin or multiple trucks
1. Export of cattle picked up from different farms in the same truck
It is possible to send a shipment of cattle comprised of animals acquired from various farms and that would be transported in the same trailer to Mexico, which means that they are covered by different export certificates and could be inspected by different veterinarians, as long as the following procedures are followed:
- Each farm of origin must have a certificate signed by a veterinarian accredited for export cattle to Mexico.
- Once the animals have been loaded from the first farm, the accredited veterinarian (or his technician) must seal the shipment. The number of animals and seal numbers must be noted on the certificate.
- The accredited veterinarian (or his technician) responsible for the subsequent farm must break the seals applied from the preceding farm. Please remember that the designation certificate to affix seals does not permit the removal of official seals. An authorization from the district office in advance is required to remove an official seal. After verifying identification and loading the animals at the second farm, the trailer is sealed. The new seal numbers are written on the health certificate that covers the animals loaded, as well as noting the seal numbers that were discarded when the vehicle was opened. The total number of animals in the truck is recorded on the certificate.
- This procedure must be repeated until the shipment arrives at the border.
2. Export of cattle in multiple truck shipment on a single certificate
It is possible to send multiple trucks under a single export certificate as long as the following conditions are met:
- The exporter is the same for all animals,
- The importer and final destination in Mexico are the same for all animals The Mexican authorities made it clear that the destination written on the certificate must be the final destination and not a temporary destination before the animals are distributed to other locations,
- The accredited veterinarian is the same for all animals,
- Each truck will have its own original transit import permit from the USDA.
The original and three copies of the official export certificate, completed as described above, must accompany the first truck, and three copies of the certificate must accompany each subsequent truck. Seal numbers applied on a truck must be recorded on the copies that accompany this truck. Accredited veterinarians must keep on file the seal numbers that have been applied to each truck.
The total number of animals in the shipment, not the total number in a specific truck, must be recorded on all copies since changes can occur when animals are unloaded and loaded again at feed and water rest stops. All trucks must pass at the U.S. border and arrive at the inspection station at the Mexico-U.S. border at the same time.
6.5 Sheep and goats (updated June 2016)
Health certification
1. General requirements described in section 6.1 must be reviewed.
2. The veterinary health certificate is the HA1385 Export of sheep or goats to Mexico/Exportación de ovinos o caprinos a México.
3. The status of the HA1385 certificate must be verified with the CFIA district office before beginning testing to ensure that the certificate is current and matches the "hoja de requisitos" and that Canada is free of diseases mentioned in article 2 and 3 of the export certificate: foot and mouth disease, Brucella melitensis, rinderpest and screwworm (Cochliomya hominivorax and Chrysomya bezziana).
4. The exporter should be advised that transits of sheep and goats to Mexico are currently prohibited by the United States Department of Agriculture (USDA). Therefore, small ruminants exported to Mexico can only be transported by air or by sea.
5. The animals must be born and raised in Canada.
6. Regulations prohibiting the feeding of ruminant origin meat and bone meal and greaves to ruminants have been enacted in Canada on August 4, 1997.
7. The animals originate from a flock/herd:
- that is free of bovine tuberculosis;
- in which no cases of bluetongue, contagious ecthyma, or campylobacteriosis (Campylobacter fetus fetus and C. jejuni) were diagnosed within 12 months prior to the date of export;
- in which no cases of enzootic abortion of ewes were diagnosed for the past two (2) years;
- in which maedi-visna and caprine arthritis and encephalitis (CAE) were not diagnosed for the last three (3) years and in which no sheep or goats of inferior health status were introduced during that period. In case of addition, a written declaration from the actual owner will be accepted to confirm that this requirement is met. This declaration will be kept on file by the accredited veterinarian;
- which is enrolled in a Voluntary Scrapie Flock Certification Program approved by the CFIA and has achieved Certified level or the exported sheep have the following genotypes: 136AA/171RR or 136AA/171QR (genotype option is only available for sheep);
- in which no case of classical scrapie was diagnosed during the five (5) years prior to the date of export;
- in which classical scrapie is not currently suspected; and
- which is not under investigation for an epidemiological link with a scrapie infected premises.
8. The exported animals were kept since birth or for the past six (6) months prior to shipment in an establishment where no case of contagious agalactia was officially reported during that period.
9. The animals being exported were not vaccinated against contagious ecthyma within sixty (60) days of export.
10. The animals being exported were kept in isolation (no nose-to-nose contact with animals not tested) on the premises of origin or assembled in a pre-export quarantine for a period of at least thirty (30) days prior to export.
11. In order to facilitate the mandatory inspection for the presence of ectoparasites at the Mexican border, the Mexican authorities (SENASICA) require all sheep and goats to be sheared within thirty (30) days of export. Animals that have not been sheared in accordance with these guidelines will be refused entry. Advise the exporter that it is his responsibility to comply with this inspection requirement.
12. The vehicles used for the transportation of the animals were cleaned and disinfected prior to their loading and the exported animals did not come in contact with other animals of an inferior health status during transportation.
Certification procedure
13. Animals must be individually identified
- For sheep, the official ear tag is a tag approved or considered equivalent by CFIA under the Livestock Identification and Traceability (TRACE) Program. These tags follow the ISO 11784 standard format with 15 digits, and may be electronic or non-electronic. The first 6 digits (124000) are not always printed on sheep tags.
- For goats, the official ear tag is a CFIA HofA (Health of Animal) tag, which must be applied to the left ear. Refer to module 2.1 Identification of Livestock for further information regarding the use of HofA tags and record keeping.
14. During the isolation or quarantine period described above, the animals were tested with negative results by a CFIA laboratory or a private laboratory for the following diseases:
- Maedi-visna (sheep) or caprine arthritis-encephalitis (goats) using an ELISA test performed at the CFIA St-Hyacinthe Laboratory (Health of Animals and Food Laboratory) or at a private laboratory that meets the requirement of Policy on the Use of External Laboratories for Export Testing and is using World Organisation for Animal Health (WOAH) approved methods (consult the district office for use of a private laboratory). This test is not required for animals that are less than one year of age;
- Epizootic abortion of ewes (sheep only) using a complement fixation (CF) test performed at the CFIA Ottawa Laboratory in Fallowfield; and
- Contagious epididymitis-Brucella ovis (sheep only), using a complement fixation test performed at the CFIA Ottawa Laboratory in Fallowfield. Option 8 b. mentioned on the export certificate can't be used and the strike out must be initialed.
Note:
Refer to the special procedure for submission to CFIA laboratories described in module 3.2 Serological Testing. A notification number for exportation to Mexico will be required (to be obtained from the district office) and a specific electronic template must be sent to the CFIA laboratory.
15. The animals were treated against ectoparasites and endoparasites within 14 days of shipment. The name of the product and the date of treatment must be recorded.
16. If scrapie genotyping (for sheep only) is used to support the certification, it may be performed on blood, and the sample must be tested in a CFIA-approved laboratory. The laboratory report must indicate that the sample was submitted by a licensed veterinarian. The sheep must be identified with an approved tag at the time of sampling and the identification number be recorded on the laboratory report. A copy of the test result must accompany the health certificate sent for endorsement to the district office.
17. The animals were inspected before departure and declared free of any clinical signs of infectious, contagious or parasitic disease including maedi visna/CAE, epizootic abortion of ewes, contagious agalactia and scrapie. The animals do not show evidence of ectoparasites, trauma, lacerations or visible swellings.
18. The exported animals showed no clinical signs of contagious agalactia on the day of inspection.
How to complete the Canadian health certificate (HA1385)
19. The accredited veterinarian must use the most recent version of the HA1385.
20. Mexican authorities do not accept hand written certificates. The certificate must be typed. A fillable pdf certificate is available through the district office. The reference number should be requested in advance from the district office, to avoid hand writing it on the certificate.
21. The accredited veterinarian must complete the export health certificate in English by entering all required information according to the directions provided above. The "Reference number" is provided by the CFIA district office. The completed health certificate shall be submitted to a CFIA veterinary inspector along with the genotyping result (if applicable) to review and, if all the requirements have been met, the certificate will be endorsed. An incomplete export certificate will be returned to the accredited veterinarian to be completed. A fee will be charged for CFIA's endorsement. Endorsed certificates will be returned to the accredited veterinarian. The health certificate is valid for 10 days from the date of inspection recorded on the certificate.
References
A copy of HA1385 is available at the CFIA district office.
6.6 Cervids (updated April 2018)
Health certificate
1. General information about exportation to Mexico mentioned in module 6.1 must have been reviewed.
2. The accredited veterinarian must use the most recent version of the export certificate. The terms and conditions of export are subject to change from time to time and without notice. The status of the Canadian Food Inspection Agency (CFIA) export certificate (HA2949) must be verified with the local district office of the CFIA prior to the commencement of testing, to ensure it is current and matches the list of conditions contained in the "hoja de requisitos" (document similar to an import permit).
3. The animals were born and raised in Canada or were legally imported into Canada and have remained in this country for at least 90 days.
4. In Canada, there are animal health regulations in place that prohibit the feeding of ruminants with meat and bone meal or greaves of ruminant origin.
5. Canada must be free of Boophilus spp. ticks
6. The farm(s) of origin had no reports of bluetongue, anthrax or other reportable disease to which the species is susceptible and are not under movement restrictions and have not been quarantined by the CFIA for an infectious disease affecting cervids.
7. The animals were free of clinical signs of infectious or contagious diseases affecting the species at inspection prior to export. The animals showed no sign of Bluetongue on the day of shipment.
Certification procedure
8. Bovine brucellosis: (delete unused option)
Either
The animals to be exported originate from a country, province or herd officially free of brucellosis (Brucella spp.).
or
The animals were submitted to an individual serological test with negative results (FPA or CF) within thirty (30) days prior to the date of export.
or
The animals are not tested and are less than 6 month of age at the day of shipment.
9. Bovine Tuberculosis: (delete unused option)
Either
The animals to be exported originate from a country, province or herd officially free of Tuberculosis.
or
The animals were individually tested with negative results by federal or accredited veterinarians for the diagnosis of tuberculosis; the test was done within the 60 days prior to export. The date of reading must be recorded.
or
The animals were not tested and were younger than one month of age
10. Transmissible spongiform encephalopathies:
- In the herd of origin there have been no cases (confirmed or suspect) of CWD during the 5 years prior to the date of exportation.
- Animals are not exhibiting nervous signs compatible with transmissible spongiform encephalopathies.
- The animals are from herds that are not under, nor have they been in contact with herds having animal health restrictions due to transmissible spongiform encephalopathies.
The animals are from herds (delete unused option)
Either
Registered in an official monitoring program for chronic wasting disease of deer (CWD) and that have been in that program for at least the last five years.
or
From a zone free of CWD. (This option needs to be struck out. There is no official free zone for CWD at the moment)
11. Bluetongue requirements are related to possible activity of the vectors.
i. Exportation during the vector free season (November 1st to May 15th). The animals:
Either (delete unused option)
a. Were in a bluetongue seasonally free zone for at least 60 days without any clinical signs of bluetongue (Eligible after December 31st). For the purpose of this certificate, the wording zone includes the entire country.
or
b. Were in a bluetongue seasonally free zone for at least 28 days prior to shipment and were subjected during the residence period to a serological test for bluetongue, with negative results, carried out at least 28 days after the commencement of the residence period. (Eligible as of November 29th).
or
c. Were in a bluetongue seasonally free zone for at least 14 days prior to shipment and were subjected during the residence period to an agent identification test for bluetongue, with negative results, carried out at least 14 days after the commencement of the residence period (Eligible as of November 15th).
ii. Exportation during the vector season (May 16 to October 31). The animals were protected from Culicoides attacks during the transport to the place of shipment and
Either (delete unused option)
a. Were protected against Culicoides bites capable of transmitting bluetongue during at least 28 days prior to export and were subjected during that period to a serological test to detect antibodies to bluetongue, with negative results, carried out at least 28 days after the introduction into the pre-export quarantine.
or
b. Were protected against Culicoides bites capable of transmitting bluetongue during at least 14 days prior to export and were subjected during that period to an agent identification test for bluetongue performed on a blood sample, with negative results, carried out at least 14 days after the introduction into the pre-export quarantine.
Note: the tests must be submitted to a CFIA laboratory. Contact the CFIA district office to obtain a notification number and to discuss the tests available. Consult section 3.2: Special procedure for submission to CFIA laboratories, applicable to samples from bovine tested in isolation (IAI notification), and from sheep and goats or cervids for export to Mexico (mandatory template).
12. Animals were treated for internal and external parasites with a product approved by the competent authority for use in animals 15 days prior to shipping. Name the active compound, concentration and dose.
How to complete the Canadian health certificate (HA2949)
13. The accredited veterinarian must use the most recent version of the HA2949 export certificate. Mexican authorities do not accept hand written certificates. The certificate must be typed, including the reference number. Fillable PDF certificates to Mexico are available from the CFIA district office. Please contact your district office for more information.
14. Sentences or paragraphs marked with exponent (1) need to have non applicable option(s) deleted.
15. All the cervids must be identified with an approved or official unique identifier which allows traceability.
16. In the column "Age", record the age in months. The breed and sex must be recorded in full on the certificate. Abbreviations are not allowed.
17. The accredited veterinarian must complete the export health certificate by entering all the necessary information with the exception of the number of animals in the shipment and the seal numbers. The completed and signed health certificate shall be submitted to a CFIA veterinary inspector to review and, if all requirements have been met, the certificate will be endorsed. An incomplete export certificate will be returned to the accredited veterinarian for completion. A fee will be charged for CFIA endorsement. The endorsed certificate will be returned to the accredited veterinarian.
18. The original and three copies of the official export certificate must accompany the shipment.
19. Animals are transported in sealed conveyance that has been cleaned and disinfected before loading. The accredited veterinarian or their technician must, on the day of export, return to the farm, supervise the loading and apply CFIA seals to the transporting vehicles, after verifying that only the animals listed on the export certificate are included in the shipment. A CFIA seal must be applied to every door on the trailer.
20. The accredited veterinarian or their technician must record the number of animals in the shipment and the CFIA seal numbers in the appropriate section on the endorsed original health certificate, and initial the appropriate section. Please repeat this information on the copies of the certificate, as well as on the copy kept in the accredited veterinarian file.
Use and control of CFIA seals
21. The export of cervids to Mexico must be done in vehicles that are sealed at the location from which the animals are being shipped. Seals must be applied by the accredited veterinarian or by a technician designated by the accredited veterinarian.
22. To perform this function, accredited veterinarians or their technicians must be designated under the Health of Animals Act to affix official seals. Contact the CFIA district veterinarian to obtain a designation certificate. Please note that this designation does not allow the removal of CFIA official seals. For each situation where official seals must be removed, the designated accredited veterinarian or technician must contact their CFIA district office in advance to obtain an authorization to remove official seals.
23. Accredited veterinarians will provide the CFIA district veterinarian with the names of any technicians able to perform the duties of sealing vehicles for the shipment of animals to Mexico. This list must be updated as soon as changes in staff are made.
24. The CFIA's Animal Health district office will provide seals for the vehicles. Seals may be allocated to an accredited veterinarian or to a veterinary clinic when more than one accredited veterinarian is employed by the same clinic.
25. The district office will keep records containing the seal numbers and the names of the accredited veterinarians or veterinary clinics to which the seals were distributed.
26. Once seals are applied to all possible exits of a vehicle transporting cervids, accredited veterinarians or their designated technicians must record the numbers on the official export certificate in the appropriate section and initial the appropriate section.
27. Accredited veterinarians are responsible to keep records of the seals that were used. Seal numbers must be matched with export certificate numbers. These records must be kept for a minimum of three years.
28. Accredited veterinarians must submit, upon request, a list of the seals used and the corresponding export certificate numbers to the CFIA's Animal Health district office. The following list can be put in a table and used to submit this information. The document can be sent by facsimile, electronic mail or regular mail.
- Accredited Veterinarian or Technician
- Seal Numbers
- Export Certificate Reference Number
- Date of Application of Seals
- Truck or Trailer License Plate Number
Inspections at U.S. ports of entry
29. The animals must be presented by appointment at the U.S. port of entry.
30. A USDA transit import permit will be required to transit the USA. The transit permit may contain additional requirements such as an insecticide treatment before the transit.
References
The export health certificate HA2949 is available at the CFIA district office.
- Date modified: