Nutrition labelling compliance test
Part 1 - Nutrition labelling compliance test
Purpose and scope
The purpose of the Nutrition Labelling Compliance Test (Compliance Test) is to provide a transparent, science-based system for assessing the accuracy of nutrient information for food in Canada.
The Compliance Test outlines the Canadian Food Inspection Agency's (CFIA) procedure for assessing the accuracy of nutrient values on food labels and in advertising via laboratory analysis. The test applies to a nutrient, including an added vitamin or mineral nutrient, that is declared in the Nutrition Facts table or is the subject of a nutrient content claim or a disease risk reduction claim. The Compliance Test also assesses whether a food bearing a nutrient content claim or health claim meets the nutrient content criteria for the claim set out in the Food and Drug Regulations.
The Compliance Test does not apply to a human milk substitute, a food represented as containing a human milk substitute, a formulated liquid diet, a meal replacement, a nutritional supplement, a food represented for use in a very low energy diet, or a minimum or maximum requirement for an added vitamin, mineral nutrient or amino acid.
Guiding principles
To achieve label information that is accurate and in compliance with the Food and Drugs Act and Regulations, the following general principles apply:
- Industry is responsible for ensuring that nutrition labelling and claims are compliant with the Food and Drug Regulations and that label values accurately reflect the nutrient content of the product.
- A suitable compliance test for the accuracy of declared nutrient values must take into consideration the inherent variability of nutrients in foods and the variability of the laboratory method using appropriate statistical analysis.
- The CFIA compliance action will take into consideration not only laboratory results, but also the health risk to the public, economic loss to consumers, past compliance history of the product and the company's quality control over the manufacturing and labelling processes.
Statistical basis
The CFIA considers that the measurement of nutrient content for compliance purposes should be based on a sound statistical framework, such that the food industry would have a high probability of a correct label value passing the Compliance Test, while the consumer would have an equally high probability that the label value accurately reflects the nutrient content of the food. To this end, the Compliance Test is comprised of three acceptance criteria applied to the results of laboratory analysis of samples obtained by using a randomized sampling plan. The statistical analysis takes into account nutrient variability in foods as well as method variability. The producer's risk (the probability that a lot with acceptable label values is erroneously rejected) and the consumer's risk (the probability that a lot with unacceptable label values is erroneously accepted) are calculated using several values for each component of variability (see statistical framework in Appendix 2).
Definitions
Lot: a collection of identically labelledFootnote 1 products produced under conditions as nearly uniform as possibleFootnote 2 and available for inspection at one time.
Consumer unit: the individual container (a primary container) or a portion of the contents of the primary container.
Sample: a collection of consumer units drawn from a lot that is representative of the lot for inspection purposes.
Composite sub-sample: a subset of consumer units from the sample that are combined and mixed to homogeneity.
Nutrient definitions
Calorie calculations and nutrient definitions are found in the Food and Drug Regulations and in Elements within the Nutrition Facts Table.
Methods of analysis
Nutrients are analyzed by appropriate methods found in the most recent edition of Official Methods of Analysis of AOAC International or other collaboratively studied methods wherever possible (Refer to Laboratory Issues in Appendix 4).
Application of rounding rules
Under the rounding rules for nutrition labelling, as set out in the tables to sections B.01.401 and B.01.402 of the Food and Drug Regulations, and summarized in rounding rules, the declared label value is a single rounded value that represents an accepted range of values. The tolerances described below are added to the minimum or maximum pre-rounded value, to obtain the compliance limit (see rounding rules and calculation of compliance limit in Appendix 3).
Classes of nutrients
Class I: a vitamin or mineral nutrient that is added
Class II: a nutrient, other than an added vitamin or mineral nutrient that is in the Nutrition Facts table or that is subject to regulations for nutrient content claims or diet-related health claims.
Sampling
At least twelve individual consumer units are taken randomly from a lot and then combined to make three composite sub-samples of a minimum of four consumer units each. The three composite samples are analyzed and the mean value of the three composite sub-samples shall be the estimate of the lot nutrient content.
Acceptance criteria for lot compliance
The lot will be considered compliant if the following criteria are met (see rationale in Appendix 2):
Criterion 1: Each of the composite sub-samples of four consumer units is within 50% of the declared value expressed as a minimum or maximum value allowed by the appropriate rounding rules, and within 50% of the regulatory requirement, if any; that is, at least half as large or at most 1.5 times as large as the declared value adjusted for rounding and as the regulatory requirement (when a minimum or maximum content is specified respectively).
Criterion 2: The mean (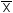 ) of the three composite sub-samples of four consumer units must fall within the minimum or maximum allowable (compliance) limit, which is the declared nutrient value or regulatory requirement, expressed as a minimum or maximum value allowed by the appropriate rounding rules, if any, with a further tolerance, if any, for that class and nutrient, applied to it (see examples in Appendix 3).
) of the three composite sub-samples of four consumer units must fall within the minimum or maximum allowable (compliance) limit, which is the declared nutrient value or regulatory requirement, expressed as a minimum or maximum value allowed by the appropriate rounding rules, if any, with a further tolerance, if any, for that class and nutrient, applied to it (see examples in Appendix 3).
Criterion 3 (Class I nutrients only): Where a vitamin or mineral nutrient is added to a food, the standard deviation (s) of the distribution of the nutrient content of the three composites of four consumer units, is compared to the mean value (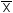 ) by ensuring that a 10% difference from the mean value lies within a 99% confidence interval for the standard deviation of the lot nutrient content. The 99% lower confidence limit evaluated as (s × 0.4344/
) by ensuring that a 10% difference from the mean value lies within a 99% confidence interval for the standard deviation of the lot nutrient content. The 99% lower confidence limit evaluated as (s × 0.4344/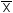 ) should be less than 0.1 or 10%.
) should be less than 0.1 or 10%.
Tolerances (Criterion 2)
Class I nutrients (no tolerance):
For added vitamins and mineral nutrients, listed in the table to section D.03.002, Food and Drug Regulations, and summarized in Foods to which vitamins, mineral nutrients and amino acids may or must be added, the declared nutrient value has no toleranceFootnote 3, i.e., the mean nutrient content of the three composite sub-samples is not less than the declared value, adjusted for rounding as in acceptance criterion 2.
Class II nutrients: (20% tolerance)
For vitamins and mineral nutrients other than Class I nutrients, protein, polyunsaturated fatty acids, omega-3 fatty acids, omega-6 fatty acids, mono-unsaturated fatty acids, carbohydrate, fibre, soluble fibre, insoluble fibre and potassium, that are declared in the Nutrition Facts table or that are the subject of a nutrient content claim or of a disease risk reduction claim:
- the regulated limit for the claim has a 20% tolerance, i.e. the mean nutrient content of the three composite sub-samples is not less than 80% of the minimum nutrient level required by the Food and Drug Regulations; and
- the declared label value has a tolerance of 20%, i.e., the mean nutrient content of the three composite sub-samples is not less than 80% of the declared value, adjusted for rounding.
For Calories, fat, saturated fat, trans fat, cholesterol, sodium, sugars and sugar alcohols, that are declared in the Nutrition Facts table or that are the subject of a nutrient content claim or of a disease risk reduction claim:
- the regulated limit for the claim has a 20% tolerance; i.e., the mean nutrient content of the three composite sub-samples is not greater than 120% the maximum nutrient level permitted in theFood and Drug Regulations; and
- the declared nutrient value has a tolerance of 20%, i.e., the mean nutrient content of the three composite sub-samples is not more than 120% of the declared value, adjusted for rounding.
Exceptions to class II
Stricter tolerances may be applied, as assessed on a case by case basis, as follows:
- Where the difference between the declared nutrient value and the compliance limit for the nutrient content of the sample may result in a label value that would be a potential risk to health, based on a health risk assessment established by Health Canada.
- Where a comparative claim is found not to be statistically valid, i.e. where providing tolerances allows the food bearing the claim to be indistinguishable from the reference food.
Overages and deficiencies - Class I and II nutrients
Where a minimum limit applies, the nutrient content of the sample may exceed the declared value by an amount that is consistent with good manufacturing practices, and provided that such an overage does not present a risk to health and is not misleading.
Where a maximum limit applies, the nutrient content of the sample may be below the declared value by an amount that is consistent with good manufacturing practices and provided that such a deficiency does not present a risk to health and is not misleading.
Use of data bases
Industry is responsible for complying with all the requirements for nutrient composition and for the accuracy of the information provided on labels. Companies may choose the risk management strategy for developing accurate nutrient data best suited to the foods to be labelled. The use of nutrient data bases is one tool within this context.
To assist manufacturers of multi-ingredient foods, the Food and Drug Regulations require that food ingredients intended solely for use in the manufacture of other prepackaged foods must provide relevant nutrition information about their product [B.01.404, FDR].
Verification of label values by the CFIA will focus on industry system controls, including record keeping, raw material control and specifications, company lab analysis, documentation of data sources, audit verification, management of ingredient data (including updates, ingredient changes, substitutions and processing effects). The CFIA will not evaluate nutrition labelling data bases, as such. The definitive determination of compliance of label values by the CFIA will be based on laboratory analysis, as outlined in the Compliance Test. A tolerance of 20% is allowed in recognition of the variability inherent in nutrient concentrations and to encourage manufacturers to label the food with the true lot average. Where variation is very high, a conservative label value would avoid exceeding the compliance limit. If any product is found to be out of compliance, the CFIA intends to work with the manufacturer to understand and correct the problem.
Implementation
The CFIA Compliance Test applies to a nutrient, including an added vitamin or mineral nutrient, that is declared in the Nutrition Facts table or is the subject of a nutrient content claim or a disease risk reduction claim.
The approach to assessing compliance of nutrient values for raw single ingredient foods for which nutrition labelling is voluntary will be reviewed when adequate data becomes available for these products.
Table - Sampling plan and tolerances
| Class | Description | Nutrients | Acceptance criterion 1, Table note a, Table note b sub-sample | Acceptance criterion 2 Tolerances Table note a, Table note b | Acceptance criterion 3, 99% confidence interval Table note d |
|---|---|---|---|---|---|
| Class I (min) Table note c |
added nutrients (e.g. added vitamin C) |
added vitamins, mineral nutrients, amino acids |
each sub-sample ≥ 50% declared nutrient value |
≥ declared nutrient value |
[(s × 0.4344) ÷ x̅] ≤ 0.1 |
|
Class II (min) Table note c |
a naturally occurring nutrient that is declared in the Nutrition Facts table and/or for which a health or nutrient content claim is made. |
protein, polyunsaturated fatty acids, omega 3 fatty acids, omega 6 fatty acids, mono-unsaturated fatty acids, carbohydrate, starch, fibre, soluble fibre, insoluble fibre, vitamins and minerals (e.g. potassium) |
each sub-sample ≥ 50% declared nutrient value |
≥ 80% declared nutrient value |
does not apply |
|
Class II (max) Table note c |
a naturally occurring nutrient declared in the Nutrition Facts table and/or for which a health or nutrient content claim is made. |
Calories, fat, saturated fat, trans fat, cholesterol, sodium, sugars, sugar alcohols |
≤ 150% declared nutrient value |
≤ 120% declared nutrient value |
does not apply |
Examples
The following examples show how the three criteria of the Compliance Test would be used to assess the accuracy of declared nutrient values from laboratory analysis data. For each scenario, the following information is shown: the amount of nutrient declared in the Nutrition Facts table and/or required for the nutrient content claim; the laboratory analysis of three sub-samples, mean value and standard deviation (s); and the assessment based on one or all of the criteria and justification.
1. Vegetable oil - Fat and fatty acids
Consider the example of vegetable oil to assess a claim about a class II nutrient: "trans fat free"
- Nutrition Facts table declarations per 10 mL: fat = 9 g, saturated fat = 0.5 g, trans fat = 0 g; claim "trans fat free".
This claim has conditions related to fat and saturated fat, so these nutrients must be assessed as well. According to the Table - Sampling plan and tolerances, maximum pre-rounded values must be used for the assessment of fat, saturated fat and trans fat.
Fat
- Laboratory results: sub-samples = 8.9 g, 9.1 g, 9.0 g; mean = 9.0 g; standard deviation (s) = 0.1 g
- Compliance limit criterion 1 for declared value:
(maximum pre-round + 50% of declared value) = [9.4 g + (0.5 × 9 g)] = 13.9 g - Compliance limit criterion 2 for declared value:
(maximum pre-round + 20% of declared value for class II) = [9.4 g + (0.2 × 9 g)] = 11.2 g - Assessment:
criterion 1: compliant: each sub-sample < 13.9 g
criterion 2: compliant: 9.0 g (mean) = 9 g (declared) (which is < 11.2 g)
Saturated fat
- Laboratory results: sub-samples = 0.62 g, 0.65 g, 0.62 g; mean = 0.63 g; s = 0.017 g
- Compliance limit criterion 1 for declared value:
(maximum pre-round + 50% of declared value) = [0.74 g + (0.5 × 0.5 g)] = 0.99 g - Compliance limit criterion 2 for declared value:
(maximum pre-round + 20% of declared value for class II) = [0.74 g + (0.2 ×0.5 g)] = 0.84 g
(see Appendix 3, Table 3) - Assessment:
criterion 1: compliant: each sub-sample < 0.99 g
criterion 2: compliant: 0.63 g (mean) < 0.84 g
Trans fat
- Laboratory results: sub-samples = 0.28 g, 0.28 g, 0.3 g; mean = 0.29 g; s = 0.01 g
- Compliance limit criterion 1:
(maximum pre-round + 50% of 0.2 g) = [0.199 g + (0.5 × 0.2 g)] = 0.3 g - Compliance limit criterion 2:
(maximum pre-round + 20% of 0.2 g for class II) = [0.199 g + (0.2 × 0.2 g)] = 0.24 g - Assessment:
criterion 1: compliant: each sub-sample ≤ 0.3 g
criterion 2: non-compliant, for Nutrition Facts table declaration and "trans fat free" claim: 0.29 g (mean) > 0.24 g
2. Lean ground beef - Iron
Consider the example of lean ground beef to assess the declaration in the NFt about a class II nutrient: Iron
- NFt declaration per 100 g serving: Iron = 1.75 mg = 10 % DV
According to the Table - Sampling plan and tolerances, minimum pre-rounded values must be used for the assessment of iron.
- Laboratory results: sub-samples = 1.4 mg, 1.5 mg, 1.6 mg; mean = 1.5 mg; s = 0.1 mg
- Compliance limit criterion 1 for declared value:
(minimum pre-round − 50% of declared value) = [1.625 mg − (0.5 x 1.75 mg)] = 0.75 mg - Compliance limit criterion 2 for declared value:
(minimum pre-round − 20% of declared value for class II) = [1.625 mg − (0.2 ×1.75 mg)] = 1.275 mg (see Appendix 3, Table 3) - Assessment:
criterion 1: compliant: each sub-sample > 0.75 mg
criterion 2: compliant: 1.5 mg (mean) > 1.275 mg
Since the declared value of 1.75 mg iron is compliant, the % DV declared must now be assessed.
- Assessment of declared % daily value (DV): For iron, the % DV is based on the rounded amount, by weight, of the nutrient, which is 1.75 mg declared.
- DV for iron as per Column 4 of Part 2 of the Table of daily values = 18 mg
- Rounded amount = 1.75 mg
(1.75 mg ÷ 18 mg) × 100 = 9.72 % is rounded to 10 % DV as per rounding rules (see Appendix 3, Table 1), which corresponds to the % DV declared, therefore compliant.
3. Granola cereal - Fibre
Consider the example of granola cereal to assess the declaration in the NFt about a class II nutrient: Fibre
- NFt declaration per 55 g serving: Fibre = 4 g = 13 % DV
According to the Table - Sampling plan and tolerances, minimum pre-rounded values must be used for the assessment of fibre.
- Laboratory results: sub-samples = 2.4 g, 3.3 g, 3.5 g; mean = 3.1 g; s = 0.6 g
- Compliance limit criterion 1 for declared value:
(minimum pre-round − 50% of declared value ) = [3.5 g − (0.5 × 4 g)] = 1.5 g - Compliance limit criterion 2 for declared value:
(minimum pre-round − 20% of declared value for class II) = [3.5 g − (0.2 × 4 g)] = 2.7 g (see Appendix 3, Table 3) - Assessment:
criterion 1: compliant: each sub-sample > 1.5 g
criterion 2: compliant: 3.1 mg (mean) > 2.7 g
Since the declared value of 4 g fibre is compliant, the % DV declared must now be assessed.
- Assessment of declared % daily value (DV): For macronutrients such as fibre, the manufacturer may calculate the % DV based on rounded or unrounded amounts, by weight, of each nutrient. Compliance of the % DV must therefore be assessed in terms of both the rounded amount of 4 g fibre declared, and the minimum pre-round value of 3.5 g fibre.
- DV for fibre as per Column 3 of Part 1 of the Table of daily values = 28 g
- Calculation based on rounded amount = 4 g
(4 g ÷ 28 g) × 100 = 14.28 % is rounded to 14 % DV as per rounding rules (see Appendix 3, Table 1). Before assessing compliance, we must also calculate % DV based on the minimum pre-round value. - Calculation based on minimum pre-round = 3.5 g
(3.5 g ÷ 28 g) × 100 = 12.5 % is rounded to 13 % DV as per rounding rules, which corresponds to the % DV declared. This is deemed compliant (since for macronutrients, the % DV may either be calculated based on rounded or unrounded amounts, by weight).
4. Pasta - Added iron
Consider the example of pasta to assess the declaration in the NFt about a class I nutrient: Added iron
- NFt declaration per 85 g serving: Iron = 2.5 mg Footnote * = 14 % DV
- Laboratory results: sub-samples = 2.42 mg, 2.51 mg, 2.47 mg; mean = 2.467 mg; s = 0.045 mg
- Compliance limit criterion 1 for declared value:
(minimum pre-round − 50% of declared value) = [2.375 mg − (0.5 × 2.5 mg)] = 1.125 mg - Compliance limit criterion 2: Class I = pre-round minimum limit (no tolerance) = 2.375 mg (see Appendix 3, Table 2)
- Assessment:
criterion 1: compliant: each sub-sample > 1.125 mg
criterion 2: compliant: 2.467 mg (mean) > 2.375 mg
criterion 3:compliant: [(0.4344 × s) ÷ mean] = [(0.4344 × 0.045 mg) ÷ 2.467 mg] = 0.008 (99% lower confidence limit) < 0.1 (compliance maximum limit)
Since the declared value of 2.5 mg iron is compliant, the % DV declared must now be assessed.
- Assessment of declared % daily value (DV): For iron, the % DV is based on the rounded amount, by weight, of the nutrient, which is 2.5 mg declared.
- DV for iron as per Column 4 of Part 2 of the Table of daily values = 18 mg
- Rounded amount = 2.5 mg
(2.5 mg ÷ 18 mg) × 100 = 13.89 % is rounded to 14 % DV as per rounding rules (see Appendix 3, Table 1), which corresponds to the % DV declared, therefore compliant.
Note:
Also meets minimum level of 2.9 mg/100 g required for iron fortification of pasta.
5. Wieners - fat-reduced
Consider the example of wieners to assess the declaration in the NFt about a class II nutrient: "fat reduced"
- NFt declaration per 55 g serving: Fat = 7 g;
According to the Table - Sampling plan and tolerances, maximum pre-rounded values must be used for the assessment of fat.
- Laboratory results: fat-reduced product, sub-samples = 7.7 g, 8.2 g, 8.0 g; mean = 8.0 g
- Compliance limit criterion 1 for declared value:
(maximum pre-round + 50% of declared value) = [7.4 g + (0.5 × 7 g)] = 10.9 g - Compliance limit criterion 2 for declared value:
(maximum pre-round + 20% of declared value for class II) = [7.4 g + (0.2 × 7 g)] = 8.8 g (see Appendix 3, Table 3) - Assessment:
criterion 1: compliant for NFt: each sub-sample < 10.9 g
criterion 2: compliant for NFt: 8.0 g (mean) < 8.8 g
Nutrient content claim: fat reduced by 25% compared to regular brand Y wieners
- NFt declaration for Brand Y wieners: Fat = 10 g
- Laboratory results: regular Brand Y wieners, sub-samples = 10.3 g, 10.4 g, 10.5 g; mean = 10.4 g,
- Regulation: fat reduction claim - must be minimum 25% reduction
- Compliance limit, class II exception, comparative claims:
regular product: 10.4 g mean or if do not have laboratory analysis, 10 g declared, pre-rounded maximum = 10.4 g;
fat-reduced product: maximum limit @ 25% reduction = 0.75 × 10.4 g = 7.8 g
Assessment Class II exceptions, comparative claims: non-compliant for fat-reduction claim: 8.0 g (mean) > 7.8 g
6. Fruit drink - Vitamin C added
Consider the example of a fruit drink to assess the declaration in the NFt about a class I nutrient: Added vitamin C
- NFt declaration per 250 mL serving: Vitamin C = 60 mg
According to the Table - Sampling plan and tolerances, minimum pre-rounded values must be used for the assessment of vitamin C.
- Laboratory results: sub-samples = 50.0 mg, 85.2 mg, 100.2 mg; mean = 78.5 mg; s = 25.77 mg
- Compliance limit criterion 1 for declared value:
(minimum pre-round − 50% of declared value) = [59.5 mg − (0.5 × 60 mg)] = 29.5 mg - Compliance limit criterion 2: Class I = pre-round minimum limit (no tolerance) = 59.5 mg (see Appendix 3, Table 2)
- Assessment:
criterion 1: compliant: each sub-sample > 29.5 mg
criterion 2: compliant: 78.5 mg (mean) > 59.5 mg
criterion 3: non-compliant: [(0.4344 × s) ÷ mean] = [(0.4344 × 25.77 mg) ÷ 78.5 mg] = 0.14 (99% lower confidence limit) > 0.1 (compliance maximum limit)
- Date modified: