Recognition of Export Grain Analysis by Authorized Laboratories (REGAL) program
On this page
Fees
For testing and sampling
REGAL authorized laboratories are responsible for setting the fees associated with testing for grain export.
If a CFIA inspector is used to sample grain for export (for containerized or railcar shipments only): Refer to CFIA Fees Notice Part 12, Table 1, Item 12 (per sample).
For a Canadian phytosanitary certificate or a Canadian phytosanitary certificate for re-export
Fees for a Canadian phytosanitary certificate or a Canadian phytosanitary certificate for re-export vary with the transaction value.
REGAL Laboratory Services Standards
For testing
- Destined for Mexico: two (2) working days from sample receipt to results issuance
- Destined for all other countries that fall within the scope of authorized testing services: three (3) working days (or agreed to with customer) from sample receipt to results issuance
For phytosanitary certificate issuance
Ten (10) business days from receiving complete application package
The REGAL program
The Recognition of Export Grain Analysis by Authorized Laboratories (REGAL) program provides a testing alternative for grain export shipments. Grain exports must be sampled and tested for percentage soil, live insects and/or weed seeds before being shipped internationally. If test results meet the requirements of the importing country, the CFIA issues a phytosanitary certificate and the shipment proceeds on its way.
To meet the demand for Canadian grain and, as a result, to test grain samples and issue phytosanitary certificates, the CFIA has established an alternative service delivery program. The REGAL program allows authorized laboratories to offer the same testing service providing more and quicker options for exporters.
Grain exporters that require live insect testing and percentage soil to any country and/or detection of weed seeds under the scope of the REGAL program on grain shipments can submit their product to an authorized REGAL laboratory. Grain shipments under the REGAL program include whole grains (not intended for propagation, for example: not for planting), split dicots or grains that have the seed coat or hull removed.
Grain exporters in Canada can choose to have their grain shipments tested by the CFIA or, for faster service, by CFIA-authorized private laboratories that are part of the REGAL program (Accredited Seed Labs or ISO/IEC 17025 laboratories that meet the program standards).
Tests outside of the REGAL scope must be conducted by the CFIA (for example, disease testing, weed seeds not included in the REGAL program, seed shipments for propagation, and crushed or ground grains). The CFIA will also remain the sole issuer of phytosanitary certificates.
Exporters are responsible for ensuring that their grain has had the correct testing to meet the importing country's requirements. Country-specific requirements may change frequentlyFootnote 1. Exporters are encouraged to confirm requirements by contacting their local CFIA office.
Authorization and oversight
Authorizing service providers
The CFIA can authorize the following types of service providers:
- Canadian ISO/IEC 17025 accredited laboratories
- Canadian CFIA-accredited seed laboratories in the Seed Laboratory Accreditation and Audit Protocol (Seed LAAP) program that have purity testing (other seed determination) for the applicable crops in their scope of accreditation
The CFIA has built standards into the REGAL program as part of its oversight role to ensure that industry is comfortable with the program and that authorized private laboratories provide the necessary level and quality of service as CFIA laboratories.
Oversight
All private authorized laboratories used will be subject to regular audits and monitoring to ensure the CFIA's grain export program objectives are met, and that they continue to adhere to Canada's export requirements.
Working with the Canadian Grain Commission
The Canadian Grain Commission (CGC) is mandated to serve producer interests by upholding the Canada Grain Act. The CGC has implemented a number of programs and safeguards to ensure the fair treatment of Canadian grain producers. It does not have a role in the REGAL program.
Roles and responsibilities
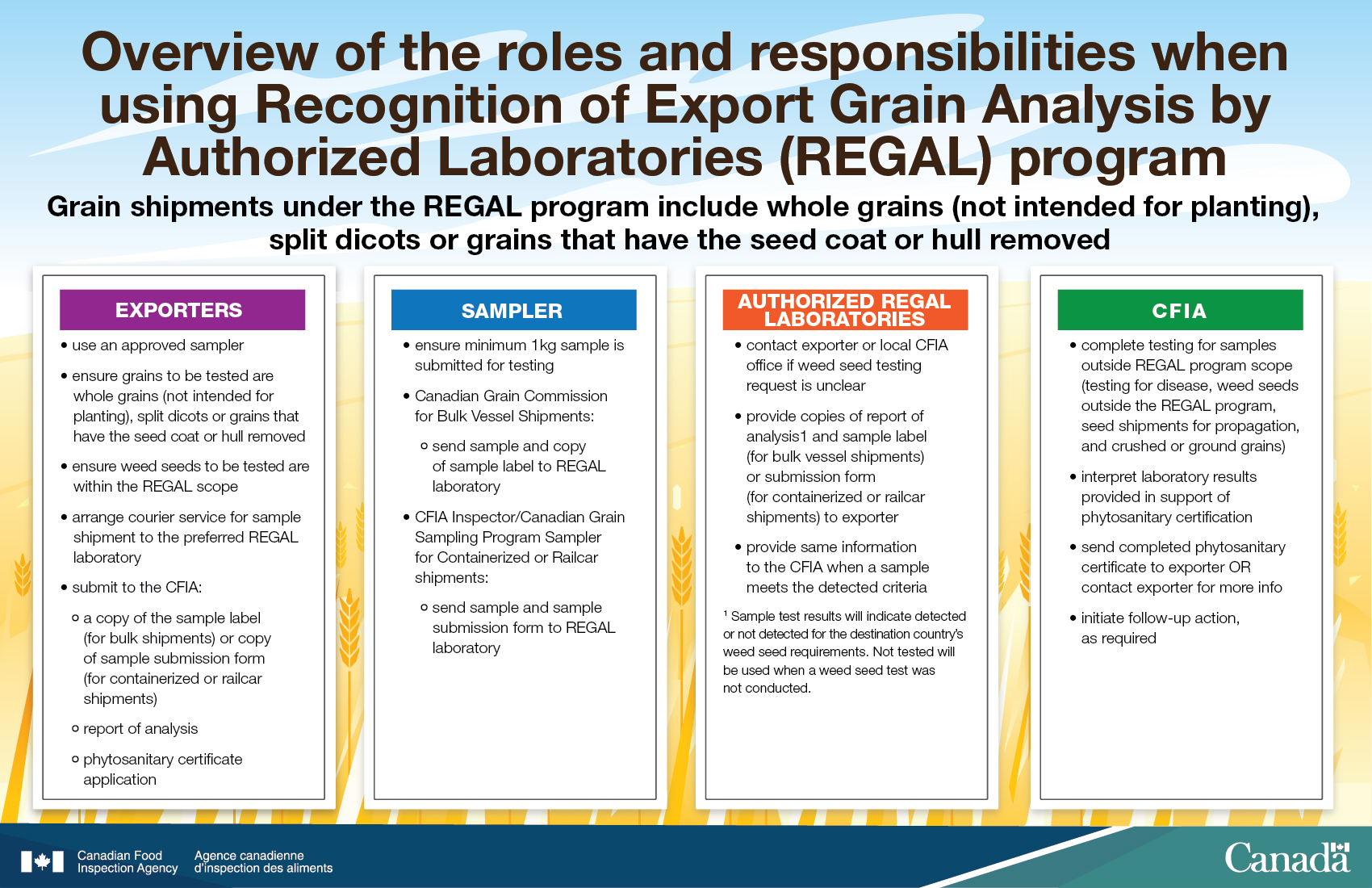
Overview of the roles and responsibilities when using the REGAL program
Grain shipments under the REGAL program include whole grains (not intended for planting), split dicots or grains that have the seed coat or hull removed
Exporters
- use an approved sampler
- ensure grains to be tested are whole grains (not intended for planting), split dicots or grains that have the seed coat or hull removed
- ensure weed seeds to be tested are within the REGAL scope
- arrange courier service for sample shipment to the preferred REGAL laboratory
- submit to the CFIA:
- a copy of the sample label (for bulk vessel shipments) or copy of sample submission form (for containerized or railcar shipments)
- report of analysis
- phytosanitary certificate application
Sampler
- ensure minimum 1kg for testing
- Canadian Grain Commission for Bulk Vessel Shipments:
- send sample and copy of sample label to REGAL laboratory
- CFIA Inspector/ Canadian Grain Sampling Program Sampler for Containerized or Railcar shipments:
- Send sample and sample submission form to REGAL laboratory
Authorized REGAL laboratories
- contact exporter or local CFIA office if weed seed testing request is unclear
- provide copies of report of analysisFootnote 2. and sample label (for bulk vessel shipments) or submission form (for containerized or railcar shipments) to exporter
- provide same information to the CFIA when a sample meets the detection criteria
CFIA
- complete testing for samples outside REGAL program scope (testing for diseases, weed seeds outside the REGAL program, seed shipments for propagation, and crushed ground grains)
- interpret laboratory results provided in support of phytosanitary certification
- send completed phyotosanitary certificate to exporter OR contact exporter for more info
- initiate follow-up action, as required
Exporter
When the sample is to be sent to a REGAL authorized laboratory for testing, it is the exporter's responsibility to:
- ensure that an approved sampler is used for sampling activities
- ensure the weed seeds to be tested for are on the REGAL weed seed list
- ensure grains to be tested are whole grains (not intended for planting), split dicots or grains that have the seed coat or hull removed
- indicate to the sampler their preferred authorized REGAL laboratory for analyzing grain samples
- arrange courier service for shipment of the sample to the preferred authorized REGAL laboratory
- arrange for the completed form to accompany the sample to the authorized REGAL laboratory
The exporter must submit the documents as detailed in the table above to the CFIA at least 10 days before the grain is shipped (for containerized and railcar shipments) or as soon as the results are available from the REGAL laboratory (for bulk vessel shipments) in order to obtain the phytosanitary certificate:
Approved samplers
- Grain must be sampled by a CFIA-approved sampler.
- The Canadian Grain Commission (CGC) is the only approved sampler for bulk vessel shipments
- CFIA inspectors or facilities and third-party samplers approved under the Canadian Grain Sampling Program (CGSP) are approved samplers for containerized and railcar shipments.
When the sample is to be collected to be sent to a REGAL authorized laboratory for testing, it is the sampler's responsibility to complete the following:
- For bulk vessel shipments
- ensure a minimum 1 kg sample is submitted to the REGAL laboratory for testing
- include a copy of the sample label that provides the necessary information for the phytosanitary certificate to be issued
- notify the exporter when the official sample is ready to be shipped to the REGAL authorized laboratory
- send the sample and copy of the sample label directly to the preferred REGAL laboratory as arranged by the exporter
- For containerized and railcar shipments
- ensure a minimum 1 kg sample is submitted to the REGAL laboratory for testing
- complete the sample submission form with information from the exporter
- notify the exporter when the official sample is ready to be shipped to the REGAL authorized laboratory
- send the sample and sample submission form directly to the preferred REGAL laboratory as arranged by the exporter
When CGSP-approved facilities or samplers collect the samples:
- the requirements of the CGSP must be followed
- the sample submission form must meet the requirements set out in the CGSP, Appendix 7
- the exporter and the sampler must collaborate to use a common unique identifier that is traceable on the sample submission form and the application for a phytosanitary certificate (for example, lot number, railcar number, etc.)
When CFIA inspectors collect the samples, they will sample the shipment, label the samples and complete a sample submission form.
Completing the sample submission form
The exporter (for bulk vessel shipments) or sampler (for containerized or railcar shipments) is responsible for completing the sample submission formFootnote 3.
Ensure the sample submission form includes the destination country and the test results required. It is important to specify which weed seeds to test for and to confirm they are on the REGAL Weed Seed list if the destination country is one other than Mexico, India, China or Vietnam on the sample submission form. Contact the local CFIA office to clarify weed seed requirements for destination country.
All REGAL program samples require a test for live insects and percentage soil and may or may not require a weed seed analysis depending on the destination country requirements.
Authorized REGAL laboratories
When a REGAL authorized laboratory receives the sample for testing, it is the laboratory's responsibility to complete the following:
REGAL Laboratory Service Standard for Testing
- Destined for Mexico: 2 working days from sample receipt to results issuance
- Other destinations within the scope of authorized testing services: 3 working days (or as agreed to with the customer) from sample receipt to results issuance
- For bulk vessel shipments
- contact the exporter or the CFIA local office if the weed seed testing request is unclear
- provide copies of the report of analysis and a copy of the sample label for the bulk vessel to the exporter
- send copies of the sample label and report of analysis to the CFIA when soil, weed seeds regulated by the destination country, or live insects are detected
- For containerized and railcar shipments
- contact the exporter or CFIA local office if the weed seed testing request is unclear
- provide copies of the report of analysis and the sample submission form to the exporter
- send copies of the sample submission form and report of analysis to CFIA when soil, weed seeds regulated by the destination country, or live insects are detected
CFIA
When the CFIA receives the required documents, it is the responsibility of the phytosanitary office to:
Service standard for issuing phytosanitary certificates
10 business days from receiving complete application package
- carefully interpret the laboratory results that are provided in support of phytosanitary certification
- send a complete phytosanitary certificate to the exporter OR contact the exporter for more information
- initiate follow-up action, as required
Additional information
- Policy on the issuance of phytosanitary certificates for export and re-export
- Canadian Grain Sampling Program
- Authorized laboratories for testing of grain exports
- Date modified: