Post-mortem examination program
On this page
- Definitions
- Objectives of MPIP
- MPIP essential elements
- MPIP training and accreditation
- Defect detection
- Defect removal and handling of off-line carcasses, carcass parts and viscera
- Poultry rejection process
- Process control(s)
- MPIP implementation and authorization
- 9.1 Step 1: Preliminary period and assessment
- 9.2 Step 2: Phase 1 – Preparatory period and assessment
- 9.3 Step 3: Phase 2 – Trial period and assessment
- 9.4 Step 4: Phase 3 – Pre-authorization implementation period an assessment
- 9.5 Step 5: Post-authorization Implementation Period and Assessment
- 9.6 Step 6: Annual re-assessment
- Poultry rejection process (PRP) implementation and authorization
Abbreviations
- Ac – Accept (such as accept number)
- AQL – Acceptable quality limit
- CDS – Carcass dressing standards
- CFIA – Canadian Food Inspection Agency
- CME – Corrective measure evaluation
- Cph – Carcass per hour
- DC – Dressing condition
- DDS – Defect detection standards
- ES – Evisceration standards
- FS – Food safety
- IBR – Incorporation by Reference
- ISO – International Standards Organization
- MPIP – Modernized Poultry Inspection Program
- NT – Not trimmable
- NTOL – Not trimmable on line
- PC – Process control
- PCP – Preventive control plan
- PE – Process evaluation
- PPV – Post-chill product verification
- PPV – Post-chill product verification
- PRP – Poultry rejection process
- PS – Presentation standards
- RCT – Rejection correlation test
- Re – Reject (such as reject number)
- SFCR – Safe Food for Canadians Regulations
- VIC – Veterinarian-in Charge
When an authorization to conduct Fundamentals of the Post-mortem Examination Program has been granted to a licence holder, the licence holder must ensure that all carcasses and parts of food animals get examined with equal confidence as those receiving CFIA Post-mortem inspection. The licence holder must ensure that all defects that pose a risk to food safety or suitability are removed. The licensed operator operating under a post-mortem examination program must be able to perform post-mortem examination similar to CFIA post-mortem inspection conducted under similar circumstances.
In general terms, "post-mortem examination program" refers to a program that must be authorized by the Canadian Food Inspection Agency and would permit a licence holder to conduct post-mortem examination of the carcasses, parts, and blood of food animals, under the supervision of a CFIA veterinary inspector. Post-mortem examination includes the detection of defects of carcass and parts along with the other elements set out in the Incorporation by Reference (IBR) document titled Fundamentals of the Post-Mortem Examination Program, incorporated by reference in the Safe Food for Canadians Regulations (SFCR).
Modernized Poultry Inspection Program (MPIP) as written in this guidance document satisfies the requirements set in the Fundamentals of the Post-Mortem Examination Program under the SFCR.
When required the licensed operator must fully assist the veterinarian-in-charge, veterinary inspector and inspector throughout MPIP operations in the establishment.
Note
- Successful implementation of post-mortem examination program involves training, inspection and examination activities which are conducted by both CFIA and licence holder independently and/or jointly.
- The CFIA activities written in this document are for the information of the licensed holder only. For regulatory compliance assessment, the CFIA reserves right to modify their activities.
- In this document the word
- "inspection" indicates MPIP activities which are to be conducted by CFIA inspection staff
- "examination" indicates MPIP activities which to be conducted by the licence holder
1. Definitions
- Modernized Poultry Inspection Program (MPIP)
- Modernized poultry inspection program is a Preventive Control Plan (PCP) and science-based inspection system that meets intent of the post-mortem examination program (SFCR) and focuses on the slaughter process within the farm to fork food safety continuum.
- Defect detection
- The act of identifying and removing viscera and carcasses with specified pathology and processing defects before and after evisceration.
- Carcass
- Poultry carcass refers to the dead body of a bird.
- Viscera
- Refers to organs which are to be examined such as lungs, liver, heart, spleen, gastro-intestinal tract (crop, proventriculus, gizzard, duodenal loop, intestines and ceca) for defect detection.
- Carcass cavity
- Refers to the interior of the opened carcass also known as abdominal cavity for defect detection. It is examined by reflecting abdominal fat pads.
- Cavity opening
- The cavity opening is defined as the pelvic opening of the carcass including the tissue between the point of the keel, the tip of the tail and the two pelvic bones.
- Cavity defect detector
- A licence holder's employee accredited to examine the internal (abdominal) cavity of carcasses after evisceration and to identify and remove carcasses with specified pathology and/or processing defects.
- Carcass defect detector
- A licence holder's employee accredited to examine the exterior of carcasses and to identify and remove carcasses with specified pathology and/or processing defects. Carcass Defect Detectors may also be referred to as "Preselectors".
- Viscera defect detector
- A licence holder's employee accredited to examine the viscera (heart and liver plus spleen and intestines in mature poultry) to identify and remove viscera, and when applicable, the corresponding carcass with specified pathology and/or processing defects.
- Pathological defect
- Carcasses with diseases or conditions which occurred while the bird was still alive. This defect occurs at the farm level or during transport to the slaughtering establishment. Examples include Cellulitis, Ascites, Cyanosis, and Emaciation etc.
- Processing defect
- Carcasses with condition (non-pathological) which is attributable to processing operation. Examples include: Inadequate Bleeding, Mutilation/Overscald, Contamination (Faecal or Bile or Ingesta) etc.
- Fowl
- Mature chicken including laying hens (which provide table eggs) and breeder flocks (which provide hatching eggs).
- Salvaging
- Hot boning of carcasses off-line so as to recover the non-defective portions.
- Veterinarian with supervisory authority (VIC)
- In general terms, "veterinarian with supervisory authority" refers to a CFIA veterinary inspector who is authorized to supervise inspection staff in an establishment and is referred to as veterinarian-in-charge (VIC) of an establishment.
- Veterinary inspector
- The Meat Products and Food Animals requirements in Part 6, Division 7 of the Safe Food for Canadians Regulations define "Veterinary Inspector" as meaning "a person who is designated as a Veterinary Inspector under subsection 13(3) of the Canadian Food Inspection Agency Act for the purposes of the Act."
- Inspector
- The Safe Food for Canadians Act defines "Inspector" as meaning "a person designated under subsection 13(3) of the Canadian Food Inspection Agency Act or paragraph 9(2)(b) of the Canada Border Services Agency Act as an Inspector for the purposes of this Act."
1.1 MPIP requirements for CFIA inspection stations
1) Veterinary disposition station
This station is CFIA's primary inspection station required to meet the Canadian (domestic) regulatory requirements at all times.
Licence holder must provide a fully equipped veterinary disposition station as a prerequisite to receiving a condemnation report from CFIA. Under the Poultry rejection process (PRP) this station will be used to assess establishment trainers, conducting Rejection correlation test (RCT). The licence holder must provide assistance when disposition and RCT are performed.
The licence holder may use this station when not in use by the CFIA.
2) Evisceration floor inspector station
This station is CFIA's primary inspection station required to meet the Canadian (domestic) regulatory requirements at all times.
The entire evisceration, dressing and chilling areas will comprise the "station" for the evisceration floor Inspector.
The evisceration floor Inspector must also have full access to following operations:
- evisceration
- dressing
- salvaging
- off-line and on-line reprocessing and reconditioning
- poultry rejection process
- chilling
3) MPIP examination/inspection stations
The licence holder must provide on-line and/or off-line inspection stations for CFIA inspection staff performing correlations or independent sampling and testing under the evisceration, presentation, defect detection and carcass dressing standards for use by the CFIA inspection staff and/or designated industry personnel.
The details of MPIP examination/inspection stations are listed under defect detection and process control sections.
Note
- The CFIA veterinarian will be present throughout evisceration operations in the establishment.
- The CFIA Inspector will maintain a permanent presence within the carcass dressing and evisceration area throughout processing operations.
2. Objectives of MPIP
- Provide the CFIA and industry personnel with the policies and procedures that contribute to the uniformity of interpretation and consistency in the implementation of the post-mortem examination program.
- Control hazards associated with food-borne pathogens during the slaughter and processing of poultry.
- Promote the proactive control (prevent, eliminate or reduce) of hazards through the implementation of a recognized PCP system in poultry slaughtering establishments.
- Facilitate the assumption by operator of the responsibility for the defect detection, defect removal, process controls and handling of defective carcasses under continuous government oversight.
- Facilitate use of objective performance standards as process control (PC) in poultry post-mortem inspection.
- Respond to changing international trade requirements.
3. MPIP essential elements
Following are the foundations for successful implementation of MPIP:
- MPIP training and accreditation
- Defect detection
- Defect removal
- Poultry rejection process (PRP)
- Process control (PC)
These are explained individually in this guidance document.
4. MPIP training and accreditation
All employees assigned on a regular basis or providing relief in poultry slaughtering establishments operating under MPIP must be accredited. The training protocol is explained in the guidance document titled Training protocol for post-mortem examination program.
It is recommended that a minimum of two (2) licence holder's employees be chosen by the licence holder to act as designated establishment trainers.
The licence holders' trainers must train and accredit a sufficient number of company employees and be available on-site for each slaughter shift for the following MPIP activities and position(s).
- Defect Detection – to be performed by "detectors"
- carcass defect detection – carcass defect detector(s) or preselector(s)
- viscera defect detection – viscera defect detector(s)
- cavity defect detection – cavity defect detector(s)
- Poultry Rejection Process – to be performed by "rejecter(s)"
- Process Control Monitoring – to be performed by "monitor(s)"
- evisceration standards monitoring
- presentation standards monitoring
- defect detection standards monitoring
- carcass defect detection standards monitoring
Note
Licence holder may choose to train and accredit same employee for multiple MPIP activities and positions.
After the licence holder employee has completed the training and is able to perform to the satisfaction of local CFIA, he/she will be considered ready to perform MPIP post-mortem activities.
5. Defect detection
As part of the PCP system, the licence holder must
- visually examine each carcass, viscera and cavity
- perform activities related to defect detection
- remove carcasses and if needed, the associated viscera from the evisceration line
- monitor defect detection and removal as per the Defect detection standards (DDS)
Following activities listed must be performed for defect detection. These have been listed in appropriate sections as below:
Detailed procedures for defect detection by defect detectors such as body position and eye movement sequence must be established by the licence holder of each establishment based on the type of equipment used and the layout of the defect detection stations.
Note
Licence holder's carcass, cavity and viscera defect detectors must be empowered to take immediate action whenever they notice a potential loss of control.
5.1 Carcass defect detection before evisceration
Carcass defect detection may also be called "preselection".
Carcass defect detectors (preselectors) must identify and remove obviously condemnable carcasses before evisceration to decrease the risks of contamination and to increase the efficiency of the evisceration process.
Preselection procedures are to be performed at the transfer point from the slaughter line to the evisceration line, or at any other point selected by the operator prior to the eviscerator(s).
The carcass defect detector is responsible for:
- examining each carcass for defects
- removing /signalling the obviously condemnable carcasses for removal
- signalling the presence of obviously condemnable carcasses that should have been removed from the line prior to evisceration according to agreed codes (if applicable)
- trimming minor defects as long as it does not affect defect detection Footnote 1
- in some cases, and under the veterinary inspector's discretion, removing defective carcasses with certain defects or remove carcass defects
Exception for turkey operations
It is generally accepted that there are few occasions in turkey processing for which preselection is not required on an ongoing basis – especially where removal of obviously condemnable birds is performed at the farm level. MPIP therefore leaves to the discretion of the operator of the turkey slaughter establishment the option of implementing preselection based on the flock sheet information.
Obviously condemnable turkey carcasses preselected by the carcass defect detector(s) need not be removed provided that such carcasses:
- are identified or marked
- are not eviscerated
- do not contact subsequent on-line employees and equipment
- are removed by the helper/trimmer after the defect detectors
Note
The CFIA will require turkey flock preselection if turkey flocks show evidence of poor health, or of other defects or have not been properly handled. Lighting and facilities for holding preselected turkey carcasses must be readily available on site.
The carcass defect detector is not required to remove carcasses. If they are properly identified for removal, these carcasses may be removed later by other on-line employees before carcass wash.
5.1.1 List of carcass defects
Carcasses affected with the following conditions are to be removed before evisceration.
See the following section for a detailed explanation of each defect.
| Carcass defects | Chicken | Fowl | Turkey | Quail |
|---|---|---|---|---|
| Ascites | X | X | X | X |
| Cellulitis (NTOLTable Note 1) and peri-cloacal cellulitisTable Note 2 | X | X | X | X |
| Dark coloured carcasses | X | X | X | X |
| Emaciation with extreme thinness | X | X | X | X |
| Inadequate bleeding (bright red carcass) | X | X | X | X |
| Pendulous crop with emaciation | X | X | X | |
| Septicaemia, toxaemia | X | X | X | X |
| Sternal bursitis, infected breast blister (NTTable Note 3) | X | X | X | |
| Xanthomatosis | X | |||
| Others: Arthritis, synovitis and valgus varus deformity with emaciation | X | X | X | X |
Table Notes
- Table Note 1
-
NTOL: Not trimmable on-line (too extensive for trimming at normal line speed)
- Table Note 2
-
Peri-cloacal cellulitis in considered to be a defect only for chicken under version 1 of the DDS (see section 8.3)
- Table Note 3
-
NT: Not trimmable (too extensive for trimming)
The following conditions may be too extensive to be trimmed (they will be condemned as "obviously condemnable") before evisceration or may be less extensive so as to be trimmable off-line in a hygienic and expeditious fashion before chilling.
| Carcass defects (NTOLTable Note 4) | Chicken | Fowl | Turkey | Quail |
|---|---|---|---|---|
| Avian keratoacanthoma | X | X | X | |
| Extensive bruising | X | X | X | X |
| Extensive dermatitis | X | X | X | X |
| Extensive mutilation, overscalding | X | X | X | X |
| Marek's disease – cutaneous form | X | X | ||
| Sternal bursitis, infected breast blister | X | X | X |
Table Note
- Table Note 4
-
NTOL: Not trimmable on-line (too extensive for trimming at normal line speed)
5.1.2 Definitions of carcass defects
- Arthritis/synovitis/tenosynovitis
- Carcasses affected with ruptured gastrocnemius tendon and/or presence of liquid and solid material within the joint are to be removed from the evisceration line if they are also emaciated.
- Ascites (water belly)
-
Carcasses showing distended or ballooned abdomen (fluid wave) are to be removed from the evisceration.
For chicken, carcasses showing distended or ballooned abdomen (fluid wave) are to be removed from the evisceration only if they show evidence of associated conditions (such as emaciation and/or dark colour and/or subcutaneous œdema).
Note
If the evaluation for the associated conditions for chicken carcasses cannot be performed on-line, all carcasses showing distended or ballooned abdomen (fluid wave) must be removed from the evisceration line for further assessment by a company detector or rejecter. If the operator has adequate facilities to segregate carcasses to prevent cross contamination, then affected carcasses without associated conditions may be put back on the line. Such carcasses must be returned to the evisceration line within 10 minutes of having been removed from the line.
Affected carcasses without secondary assessment performed by the operator (not showing associated conditions) presented to the CFIA veterinary inspector will be disposed of by the operator and will not be included on the condemnation / rejection reports.
- Avian keratoacanthoma
- This skin condition is a formation of deep crater-shaped ulcers mainly on the back. Remove the carcass if the affected skin area is too large to trim on-line.
- Cellulitis
- Thickened, yellow coloured skin (may be with a honeycombed appearance). Remove chicken carcasses with Peri-Cloacal Cellulitis. Chicken carcasses with skin lesions smaller than 2 cm × 2 cm, including lesions on the legs and the wings of any dimension, may be passed if included within the operator's PCP system. Remove turkey and fowl carcasses with extensive Cellulitis lesions. The veterinary inspector will determine the criteria for the size of lesions which may be trimmed on-line for turkeys. Carcasses with scratches with only slight thickening and yellowing of the skin, not affecting underlying tissue, can be trimmed on-line.
- Dark coloured carcasses (Cyanosis)
- Carcasses with a dark blue-purple colour are to be removed from the line. Mild to moderately blue carcasses should be passed if the darker discolouration is the only significant finding, such as, not emaciated. Carcasses with extremity petechiation ("blood spots"), but are otherwise normal, should be passed.
- Emaciation
- Carcasses with extreme thinness and that are dark coloured must be removed from the line. Carcasses which are small (but with good finish or fleshing) may also be culled by the establishment detectors but are to be considered as a plant reject.
- Extensive bruising
- Carcasses must be removed if the affected area is too large to be trimmed on-line.
- Inadequate bleeding
- Carcasses with deep to brick red colour (head may still be attached or incomplete or no neck cut). For carcasses which are mildly red/blue, refer to the definition of Dark Coloured Carcasses (Cyanosis).
- Marek's Disease (Cutaneous Marek's)
- Enlarged feather follicles often with yellowish coloured surrounding skin. Remove the carcass if the affected skin area is too large to trim on-line.
- Mutilation
- Extensive crushing and/or deformation too large to be trimmed on-line.
- Overscald
- Damaged skin/muscle too large to be trimmed on-line caused by an over scalding.
- Pendulous crop
- Carcasses are to be removed from the line only if affected with extensive pendulous crops (representing a risk of contamination), or if associated with poor carcass condition (emaciated), or if the carcass has a bad odour.
- Sternal bursitis / infected breast blister
- Usually found in the breast or the keel area, sternal bursitis may be the result of a skin infection or a pectoral cyst. Remove the carcass if the affected area is too large to trim on-line.
- Xanthomatosis
- Thick yellowish swellings may be present in the wattles, breast, abdomen and legs. The swelling may become a pendulous mass filled with a honey coloured liquid. Remove the carcass if the affected area is too large to trim on-line.
5.2 Viscera defect detection
Depending on the speed of the evisceration lines, cavity and viscera defect detection may be performed:
- on a unit-by-unit basis where each defect detector examines the cavity and the viscera of the carcasses presented for examination; or
- on a sequential basis, where all the cavities are examined by specific cavity detectors and all the viscera are examined by a separate viscera defect detector
The operator may position the viscera defect detector(s) either before or after the cavity defect detectors. However, viscera sets must be presented with their corresponding carcasses throughout the viscera defect detection and the defect detection standards (DDS) testing zones.
In descending order, the viscera defect detector's priorities are to:
examine each viscera for defects
Note
For fowl, palpate the duodenum and/or perform other procedures which are effective for detecting all carcasses with Adenocarcinoma.
- signal carcasses for removal according to agreed codes (if applicable)
- signal the presence of obviously condemnable carcasses that should have been removed from the line prior to evisceration
- in some cases, and under the veterinary inspector's discretion, remove defective carcasses and/or viscera from the line if there is no helper/trimmer
Contamination on the viscera and pathological conditions only affecting the viscera (not affecting the carcass) are not to be counted as a defect if the operator is either not harvesting edible viscera or if an effective program is included within a PCP system which ensures that contaminated viscera are not harvested as edible.
5.2.1 List of viscera defects
| Viscera defects | Chicken | Fowl | Turkey | Quail |
|---|---|---|---|---|
| Adenocarcinoma | X | |||
| Airsacculitis | X | X | X | X |
| Contamination such as faecal, bile, ingesta, extraneous material | X | X | X | X |
| Emaciation as visible on heart, gizzard | X | X | X | X |
| Hepatitis | X | X | X | X |
| Lymphoid leukosis | X | |||
| Marek's disease – visceral form | X | X | ||
| Peritonitis | X | X | X | X |
| Septicaemia, toxaemia | X | X | X | X |
| Other conditions such as osteomyelitis, tumors | X | X | X | X |
5.2.2 Definitions of viscera defects
- Emaciation
- Extremely thin carcasses in poor conditions. The remaining fat, on the heart and gizzard, is in a moist, pinkish, sticky and jelly-like consistency
- Hepatitis
-
Carcass and viscera are to be removed if the liver exhibits multiple visible white/yellow or green-black spots of any size or shape. In turkey, only the liver is to be removed.
The liver only is to be removed if it is green and enlarged and firm (hard), or if the liver displays multiple pinpoint red spots, or if the liver displays evident signs of ascites (bosselated, cobblestone) with or without pinpoint red spots (petechial haemorrhages).
Note
Carcass and viscera are to be passed if the liver has a normal size, sharp edges, (regardless of the colour of the liver), or if the liver exhibits signs of a fatty liver (light brown, yellowish) even though they are enlarged.
- Marek's disease – visceral form
- If visceral tumours (white nodules) are present, the carcass is to be removed from the line.
- Peritonitis
- Inflammation of the lining of the abdominal viscera often seen with red tags, as a whitish to yellow, opaque, cheesy exudates, and with an off odour.
- Septicaemia or Toxaemia
- These are the acute conditions which may present various signs, for example, haemorrhages on single or multiples organs and in the cavity, congestion of various organs. In those cases, the veterinary inspector should be consulted to identify the cause of the identified lesions.
- Tumors (Leiomyoma and Hemangioma)
- The benign growth found in the meso-salpinx (membrane enveloping the oviduct) is very common and is not considered malignant. Carcasses with tumours such as Leiomyoma and Hemangioma must be left on the evisceration line. However, the viscera must be removed and discarded.
5.3 Cavity defect detection
Depending on the speed of the evisceration lines, cavity and viscera defect detection may be performed:
- on a unit-by-unit basis where each defect detector examines the cavity and the viscera of the carcasses presented for examination; or
- on a sequential basis, where all the cavities are examined by specific cavity detectors and all the viscera are examined by a separate viscera defect detector
The operator may position the viscera defect detector(s) either before or after the cavity defect detectors. However, viscera sets must be presented with their corresponding carcasses throughout the viscera defect detection and the defect detection standards (DDS) testing zones.
The cavity defect detectors must be positioned before cavities are vacuumed and prior to the internal/external carcass washer unless the operator uses an approved on-line reprocessing and reconditioning process, as explained in the Poultry Off-line and On-line Reprocessing and Reconditioning Procedures.
The cavity defect detector is not required to remove carcasses signalled for removal. If they are properly identified, these carcasses may be removed later by the helper/trimmer or by other on-line employees.
In descending order, the cavity defect detector's priorities are to:
- examine each cavity and abdominal opening for defects (such as, faecal contamination, Peri-cloacal cellulitis)
- signal carcasses with specified defects for removal or trimming according to agreed codes (if applicable)
- if applicable, function as presenters (if the operator elects to combine presentation and cavity detection duties) to permit examination of the entire cavity in establishments with eviscerator(s) which do not separate the viscera from the carcass (if applicable)
- signal the presence of obviously condemnable carcasses that should have been removed from the line prior to evisceration
5.3.1 List of cavity defects
| Internal cavity defects | Chicken | Fowl | Turkey | Quail |
|---|---|---|---|---|
| Airsacculitis | X | X | X | X |
| Contamination such as faecal, bile, ingesta, extraneous material, intestine/cloaca attached | X | X | X | X |
| Peri-cloacal cellulitis | X | X | X | |
| Salpingitis | X | X | X | X |
| Other conditions such as, odour, tumours, granuloma in quail | X | X | X | X |
5.3.2 Definition of cavity defects
- Airsacculitis
- All carcasses with liquid or solid material in the air sacs or in the lungs, left inside the cavity, measuring greater than 3 mm (5 mm for turkey) are to be removed. Carcasses with lesions that are very well capsulated by a very thick membrane of the air sacs must also be identified.
- Peri-cloacal cellulitis
- Thickened, yellow coloured skin. Remove carcasses with cellulitis lesions on the peri-cloacal area.
- Contamination
-
Contamination of the carcass cavity and/or viscera may come from different sources:
- Faecal contamination: Any visible material determined to be from the lower gastrointestinal tract within the abdominal cavity.
- Ingesta: The undigested contents of the crop, gizzard or proventriculus (liquid or solid) which have contaminated the carcass cavity. Dry and localized ingesta covering an area of a dime or less or a few isolated grains will not be considered as a defect if the operator is not performing on-line reprocessing.
- Bile Contamination: Bile stains causing a discolouration of affected tissue.
- Extraneous material: Grease stains or other foreign material within the abdominal cavity.
- Intestine/Cloaca: Refers to a length of intestine/cloaca attached to the carcass or inside the cavity and is associated with evisceration lines equipped with a new technology system. The cause is improperly adjusted equipment; the length of intestine/cloaca still attached to the carcass or inside the cavity will contaminate the internal cavity with faeces or, if it enters the giblet harvesting process, it will spread faecal contamination onto both equipment and product.
Also see Controls on contamination.
- Salpingitis
- This is an infection of the oviduct or salpinx, the reproductive organ of pullets. It is characterized by the presence of liquid or solid material, which is usually yellowish in colour. Very often the tissues surrounding the salpinx become viscous. All viscera exhibiting such lesions must be removed from the evisceration line. Any presence of solid or liquid material within the salpinx observed during examination must be recorded as a defect.
- Tumours
-
Any enlarged abnormal irregular mass of tissue in the internal cavity.
In Quails, yellowish granules of various sizes (1 mm to 15 mm) located in the air sacs or attached to abdominal cavity (granuloma).
5.4 Defect detectors station and training & accreditation station
Adequate on-line space is required for each carcass/viscera/cavity defect detector work station.
On-line space (1 meter) downstream from carcass defect detectors and prior to the venting machine for CFIA staff/licence holder to train and accredit the establishment trainers as carcass defect detectors must be provided. The space is also provided for the training and the accreditation of the carcass defect detectors by the accredit trainers.
6. Defect removal and handling of off-line carcasses, carcass parts and viscera
The licence holder may choose to remove defects (pathological and processing) detected by defect detectors either on-line or off-line as long as the defects are hygienically removed and does not lead to cross contamination.
Carcasses
Carcasses removed from any point along the evisceration line must be submitted to a post mortem examination (defect detection, rejection) unless they are rejected by the operator.
| Condition of the carcass | Action to be taken |
|---|---|
| Normal carcass | Return to the evisceration line |
| Carcass with localized pathology | Send for off-line salvaging, trimming or reconditioning or for on-line reconditioning |
| Carcass with processing defects | Send for off-line salvaging, trimming or reprocessing or for on-line reprocessing |
| Carcass suspected of having generalized pathology diseases or conditions | Send for detailed veterinary inspection or examination by a rejecter under the poultry rejection process |
Note
Depending upon the nature of the pathological and processing defect, the CFIA veterinary inspector may require additional actions to be taken in addition to above options.
Carcass parts and viscera
The carcass parts and viscera removed from evisceration line must be also handled using similar principles as used for carcasses.
Exports
Foreign countries may require additional export procedures to be conducted.
For products eligible for export to the United States, refer to the procedure concerning carcasses removed from the evisceration line outlined in Annex E: Requirements applicable to poultry abattoirs.
7. Poultry rejection process
The MPIP requires a licence holder to perform a post mortem examination of carcasses and to sort defective carcasses based on the criteria specified in this section.
The Poultry rejection process (PRP) involves the operator assuming responsibility, as instructed by the veterinary inspector, for rejecting poultry carcasses, parts and associated viscera with "certain deviations" should be discarded as rejected. These deviations are explained in section 7.2
The licence holder's rejecters must be available and provide help to the CFIA veterinary inspector on veterinary stations and during Rejection correlation test (RCT) performed by the CFIA veterinary inspector.
The licence holder must assist CFIA with compliance evaluation of the rejection process throughout slaughter and evisceration operations.
PRP specific definitions
- Condemnation
-
The term condemnation is reserved for all carcass and its parts condemned as a result of a CFIA veterinary diagnoses and disposition.
- Rejection
-
The term rejection means all carcass and its parts rejected or discarded by a trained and accredited employee performing rejections under poultry rejection process (PRP).
- Suspect carcass
-
For purpose of PRP, a suspect carcass means a carcass that has been identified by the defect detectors for removal from the line for evaluation by rejecter.
- Rejected carcass
-
A rejected carcass is a carcass showing pathological defects that should be discarded as inedible products according to the rejection criteria.
- Passed carcass
-
A passed carcass is an eviscerated carcass that was removed from the line by a defect detector, given a secondary examination by the rejecter and subsequently passed by rejecter (the carcass must be evaluated by the CFIA and localized defects must be removed).
- Questionable carcass
-
Carcasses that have been examined by a rejecter, but the rejecter is unsure if the carcass should be rejected or passed (set aside for evaluation by a CFIA veterinarian).
- Edible stream
-
The continuum of processing steps from the receipt of live birds to the shipping of poultry products accepted as edible for human consumption.
7.1 Position of the PRP station
The existing CFIA Veterinary stations may be used as PRP station. If the licence holder would like to have additional stations for sorting, they will be located closer to the existing CFIA Veterinary disposition stations.
7.2 PRP defects
Pathological defects to be managed by the trained rejecters
For the purposes of the PRP, carcasses showing "certain deviations from normal appearance" are defined as carcasses with deviations and are included within the following table:
| Codes | Deviations | Diseases and Conditions |
|---|---|---|
| 901 | Respiratory conditions | Airsacculitis |
| 902 | Sub-cutaneous conditions | Cellulitis |
| 903 | Leg conditions | Arthritis, synovitis, valgus varus deformity |
| 904 | Skin conditions | Marek's disease – skin form, Avian keratoacanthoma, dermatitis |
| 905 | Abdominal oedema | Ascites |
| 906 | Liver conditions | Hepatitis, icterus/jaundice |
| 907 | Emaciation | Extreme thinness associated with another disease or condition such as Pendulous Crop |
| 908 | Dark coloured carcasses | Cyanosis |
| 909 | Others | Marek's Disease (visceral form), pericarditis, peritonitis (fowl & turkeys), pendulous crop, adenocarcinoma (fowl) |
The deviations listed in the preceding table have been selected as they are easily identifiable by licence holder's employees trained and accredited using the training material and accreditation protocol developed by the CFIA. These are explained in disposition document (to be published) and/or within the applicable training modules as developed by the CFIA for use by licence holder. Designated industry rejecters must be trained and accredited prior to performing rejections as described in Training protocol for post-mortem examination program.
The code assigned to each disease and condition is intended for use by the operator and the veterinary inspector when issuing condemnation/rejection reports and when collating and submitting condemnation/rejection data on a monthly basis to headquarters.
Processing defects to be managed by the trained rejecters
Carcasses with processing defects, as described in poultry rejection process are to be identified by the industry designated defect detectors and re-routed to appropriate processes and/or disposed of according to the rejection requirements. These affected carcasses are not considered under the PRP and are to be handled by the operator under CFIA oversight.
Carcasses listed in the following table are processing defect which are to be handled by the licence holder. These defects are:
- not part of the rejection process
- to be handled by a designated industry employee
- not to be presented to the CFIA (unless requested by the veterinary inspector on a case-by-case basis)
| Number | Name of condition |
|---|---|
| 1 | Extensive bruising |
| 2 | Extensive contamination such as faecal, bile, ingesta |
| 3 | Extensive fractures |
| 4 | Extensive mutilation |
| 5 | Extensive overscald |
| 6 | Found dead – dead on arrival |
| 7 | Inadequate bleeding |
| 8 | Loss of identity |
Note
Only "Extensive bruising", "Found dead" and "Inadequate bleeding" are to be reported to the CFIA veterinary inspector for the monthly roll-up.
PRP defects to be submitted for CFIA veterinary inspector
Please note at all times the veterinary inspector retains discretionary powers such as instructing an operator not to submit carcasses showing certain deviations.
The carcasses and associated viscera must be referred and presented to the CFIA veterinary inspector as below:
- Carcasses are not falling within categories as explained in preceding tables titled Nine (9) Categories and Corresponding Diseases and Conditions for the PRP and Processing defects to be handled by the operator.
Questionable carcasses must be referred to the veterinary inspector for a detailed veterinary inspection and instructions.
Examples include: Anemia, Botulism, Cannibalism, Coligranuloma, Emphysema, Frostbite, Leucosis sarcoma group, Gout, Osteomyelitis, Urolithiasis, Septicaemia, etc.
- The operator is required to refer to the veterinary inspector whenever the company rejecter encounters a lot with an unusual pathology or a flock with a high rejection rate.
- In addition, at any time, a veterinary inspector may require that all rejected carcasses be submitted for detailed Veterinary inspection from a particular lot or lots.
7.3 Rejection process procedure
The rejection process involves 3 steps:
- Defect detection by defect detectors
- Evaluation and sorting (for RCT) of suspect carcasses by the rejecter
- Sorting and handling of rejected carcasses, passed carcasses and questionable carcasses by the rejecter
Carcasses and viscera showing processing defects are to be discarded as operator rejects and are not considered under the PRP as they do not involve a pathological condition. However, for animal welfare concerns, CFIA requires that the operator record the number of carcasses discarded for the following reasons: extensive bruising, found dead – dead on arrival and inadequate bleeding. These numbers are published in the Condemnation Reports on the Agriculture and Agri-Food Canada website.
Role of defect detector
- The rejection process must be initiated on each evisceration line after defect detection and related activities:
- Carcass defect detector(s) remove obviously condemnable carcasses prior to evisceration for evaluation and sorting (for RCT) by a trained and accredited rejecter.
- After evisceration, viscera defect detectors identify viscera that have specified defects that may warrant rejection of the carcass and the corresponding viscera. Each such viscera and the corresponding carcass are to be removed from the evisceration line for evaluation and sorting (for RCT) by a trained and accredited rejecter.
- Carcasses with only cavity defects are not to be submitted to the rejecter. Carcasses with localized cavity defects must be handled as per on-line/off-line reprocessing/reconditioning and/or salvaging processes.
Role of rejecter
- Suspect carcasses and associated viscera when removed from the evisceration line for suspected generalized pathology must be evaluated and sorted off-line (for RCT) by a rejecter into three groups as follows:
- rejected carcasses
passed carcasses (subject to assessment by the CFIA and removal of any localized defect(s))
Passed carcasses can be:
- eviscerated (off-line or on-line) and examined by cavity and viscera defect detectors
- accepted as is
- accepted subject to removal of any localized defect(s)
- "questionable" carcasses (set aside for the CFIA's veterinary inspector assessment).
- Each rejected carcass must be recorded by the rejecter. The rejecter also classifies (sorts) each rejected carcass under one of the nine (9) categories listed in section 7.2. Carcasses which are passed by the rejecter must be presented to the CFIA as described in section 10.4. These are the same categories that appear on the industry "Condemnation/Rejection Report for Poultry".
- If a rejecter is not sure if a carcass should be rejected or passed it is a questionable carcass. (such as a new or unknown pathology or unknown signs of pathology). Such carcasses must be set aside in a designated location approved by the VIC. All questionable carcasses and "deviations from normal appearance" not listed in the section 7.2 must be referred to the CFIA for a Veterinary judgment and disposition.
Role of CFIA veterinary inspector
The CFIA veterinary inspector will work with the rejecter on rejected carcasses to:
- assure that questionable carcasses are minimized
- provide feedback to the rejecter as part of a learning experience
- provide Veterinary assessment on rejection certificates
See section 7.8 for details.
Role of CFIA evisceration floor inspector
The CFIA Evisceration Floor Inspector monitors the rejection process (when CFIA veterinarian is not present on evisceration floor), salvaging and off-line reprocessing / reconditioning operations throughout evisceration operations.
See section 7.8 for details.
Information for producers
National and regional data for disposition of chickens and turkeys will continue to be posted at the Poultry and Egg Market Information – Canadian Industry of Agriculture and Agri-Food Canada.
7.4 PRP sampling frequency, sample size and acceptance criteria
The rejection process will be monitored by the CFIA veterinary inspector using the Rejection Correlation Test (RCT).
Sample Size
For the purposes of an RCT a lot is considered to be comprised of one truck load or about one hour's production of live poultry.
Sampling Frequency
The CFIA veterinary inspector will conduct an RCT at a minimum frequency of once per half shift as described below. The veterinary inspector reserves judgment to increase the sampling frequency.
Acceptance criteria using rejection correlation test (RCT)
The CFIA veterinarian will perform an RCT with the rejecter using false positive and false negative carcasses criteria on each lot of poultry as described in the following four (4) steps.
False positives are carcasses that would have been rejected by the rejecter but should have been passed (unwarranted rejections).
False negatives are carcasses that would have been passed by the rejecter but should have been rejected (passed in error).
The RCT will be performed as below:
Step no. 1 – Carcasses with suspected generalized pathology, which are removed from the line by the defect detectors, must be presented to a CFIA veterinary inspector by the rejecter in a manner acceptable to the VIC (either manually or on CFIA accepted facilities). Such carcasses must be:
- presented one at a time to the veterinarian
- presented in a manner to clearly indicate if the rejecter wants to reject or pass the carcass
- if rejected, placed into one of the nine (9) categories listed in the table in the section 7.2
- if not rejected, directed to the evisceration line, salvaging or off-line reprocessing/reconditioning
- if the rejecter is not sure whether the carcass should be rejected or passed, the rejecter must indicate that the carcass is a "questionable" carcass
A procedure should be in place to coordinate the work of the rejecters and the CFIA veterinary inspector in an efficient and timely manner.
Step no. 2 – The CFIA veterinary inspector will inform the rejecter of the correct name and disposition for each false positive, false negative and questionable carcass.
Note
Please note that whenever the rejecter correctly indicates that a carcass should be rejected, but places it in the wrong category, the rejecter will be informed that it is a mistake and will be informed of what the correct name/category is, but such a mistake will not be counted as a false positive. Repeated mistakes should be reported by the CFIA veterinarian to the plant operator for follow-up by a plant trainer.
Step no. 3 – Rejecters must collect the number of carcasses that would have been rejected and collate the rejection results under each of the nine (9) categories listed in the table in the section 7.2. This information must be provided to the CFIA veterinarian. The veterinarian will verify if the information provided by the rejecter (number of carcasses in each category) correlates with similar information collected by the veterinarian during the RCT.
The operator must put in place a system (manual or electronic) to collect the number of carcasses rejected and the category into which each rejected carcass is placed by the rejecter.
Step no. 4 – The following information will be recorded by the CFIA veterinarian for entry into an Excel spreadsheet for every lot:
- number of carcasses rejected for pathology;
- number of false positives and the correct category/condition for each false positive;
- number of false negatives and the correct category/condition for each false negative;
- number of questionable carcass(es);
- number of birds slaughtered; and
- initials of the plant rejecter(s) and the veterinarian.
Note
CFIA will provide recommendations to its veterinary inspector(s) to track information on the type and number of questionable carcasses separately to enhance training of the rejecters.
The rejecter must dispose of the carcass as instructed by the veterinary inspector and, if rejected, must record the carcass in the correct category. CFIA will require the licence holder to share the rejection data monthly with CFIA. This data is collected by the CFIA on behalf of Agriculture and Agri-Food Canada and is used to generate CFIA's monthly "Ante Mortem and Post Mortem Inspection Report".
7.5 PRP process control
The CFIA has developed a Statistical Process Control (SPC) which is based on a Shewart Chart as incorporated into the Excel spreadsheet. Each data point on the Shewart Chart is comprised of the number of correlation errors (comprised of false positives and negatives) from 40 consecutive RCTs (about one month's production).
During the RCT, the number and distribution of false positive and negative rejected carcasses are entered into the Excel spreadsheet and PRP is assessed by evaluating the graphs generated by an Excel spreadsheet. The graphs are automatically updated whenever the results of an RCT are entered into the corresponding data entry chart within the Excel spreadsheet.
The data entered into the PRP spreadsheet generates Central Line (CL) and Upper Control Line (UCL).
Process under control
A rejection process that is under control is a process where the number and distribution of false positives and negatives during Phase 3, Pre-authorization Implementation Period, and thereafter during the Post Implementation period, reflects that which occurred during Phase 2, Trial Period, for implementing the PRP.
The PRP process is considered under control when Excel spreadsheet shows
- no data point exceeding the Upper Control Limit (UCL)
- less than seven consecutive data points located below the Central Line (CL)
Process out of control
The PRP process is considered out of control when Excel spreadsheet shows
- data point exceeding the Upper Control Limit (UCL)
- seven consecutive data points located above the Central Line (CL)
Such data points would be a strong indicator that the licence holder may have lost control over rejections.
7.6 Defect log
There is no decision tree for the PRP and no industry specific PRP specific defect log.
Instead the PRP process is controlled by CFIA veterinary inspector using a Shewart chart. CFIA starts collecting data from PRP Phase-2, Trial period onwards as explained in section 10.3.
7.7 Process out of control – action to be taken
When the process is deemed to be out of control by the CFIA, the licence holder must perform an investigation, determine the probable cause(s), submit an acceptable written corrective action plan, and take effective corrective and preventative action(s).
In addition, rejecters must be retrained and/or replaced or rejected carcasses are to be discarded as licence holder rejects, if one of the following occurs:
- repetitive errors are made by rejecters
- total number rejected or number of carcasses indicated by the rejecter(s) under each category indicated on the licence holder's condemnation/rejection report is inaccurate on an ongoing basis (repetitive occurrence) based on the RCT
- carcasses are rejected by non-accredited rejecters
Note
If the rejection process goes out of control as shown by the SPC chart generated by the PRP Excel Spreadsheet, CFIA veterinary inspector will check off the box indicating that the process is not under control on the CFIA/ACIA 5639 "Poultry Rejection Process Control Evaluation Report" form. CFIA veterinary inspector will continue to check off the box indicating that the process is not under control until an acceptable corrective action plan has been received by the VIC and the process is brought back under control as shown by the SPC chart (such as if the last data point exceeded the UCL and the new datapoint is below the UCL, or if the previous seven consecutive datapoints exceeded the CL and the new datapoint is below the CL).
PRP trend analysis
The number of false positives and negatives should tend toward zero over time, with the understanding that the zero value is the objective but is out of reach under normal operating conditions.
CFIA veterinary inspector will consult with the licence holder when there is compelling evidence that an upward trend, as shown by the various graphs generated by the Excel Spreadsheet or a cluster of false positive and/or false negative rejected carcasses, can be attributed to a cause other than normal variation in the rejection process.
False positives
Each incident of an unwarranted rejection (false positive) will be assessed on a case-by-case basis by the CFIA veterinary inspector conducting the RCT. The CFIA veterinary inspector will inform the industry rejecter each time a false positive occurs during a RCT. If warranted, the CFIA veterinary inspector may require the licence holder to replace and/or retrain a rejecter, slow down the evisceration line or add extra rejecters. Alternatively, the licence holder may elect to absorb the loss of all rejected carcasses as "plant rejects".
False negatives
There are two (2) types or origins of false negative carcasses:
- defects that are missed by the defect detectors; and
- carcasses passed in error by the rejecter.
Carcasses missed by defect detectors are controlled as part of the DDS program as described under Process Controls.
Each carcass and corresponding viscera removed by the defect detectors for suspected generalized pathology of farm origin, and not rejected by the rejecter, must be inspected by the CFIA Evisceration Floor Inspector. If the Inspector determines that the carcass and viscera may be a false negative, then the carcass and viscera must be set aside for a detailed Veterinary inspection. The CFIA veterinary inspector will inform the rejecter whenever the carcass set aside is determined to be a false negative.
Questionable carcasses
Each questionable carcass provides an opportunity for the CFIA to provide immediate feedback to the rejecter on-site and to ensure an enhancement of the knowledge and expertise of the rejecters. As rejecters gain experience in performing rejections, the number of questionable carcasses set aside should decrease and will indicate that rejecters are becoming more and more proficient in their duties. Questionable carcasses are also important because they alert the VIC of new emerging conditions.
Repetitive submission of questionable carcasses for the same reason/condition and/or unwarranted questionable defective carcasses by either one or several rejecters will be brought by the official veterinary inspector to the attention of the establishment trainer(s).
Conditions found in these questionable carcasses should be factored into subsequent accreditation and/or re-accreditation of the rejecters.
Specific circumstances
Under all of the following specific circumstances, it is to be noted that the CFIA veterinary inspector may require that all carcasses suspected of a generalized pathology and removed from the evisceration line for further rejection be submitted for a detailed veterinary inspection.
Lack of a competent rejecter
Whenever the licence holder lacks an employee listed on the current roster of trained and accredited rejecters for a slaughter shift (such as due to sickness or a severe storm that prevents the rejecters from getting to the establishment), the licence holder may:
- suspend slaughter operations until a competent rejecter can be brought in
- all carcasses sent by the detectors for assessment by the rejecter (for example, suspected of having generalized pathology) are discarded as licence holder rejects
- other options acceptable to the CFIA
Lots with an unusual pathology
When either defect detectors and/or rejecters are confronted with a new or unusual condition/pathology, Veterinary assistance must be sought. Once the condition is identified, the disposition will be communicated to the rejecter(s) and the CFIA Evisceration Floor Inspector. If carcasses affected with the identified condition are rejected, they should be recorded as per VIC guidance in the licence holder's database.
Flocks with a high rejection rate
Outlier flocks (with a high rejection rate) are generally anticipated before they are processed through the review of the advance copy of the flock sheet and of the producer profile in the licence holder's database. The licence holder must take appropriate action before the lot reaches the evisceration floor.
As soon as an unusually high rejection rate is experienced by the rejecter(s) for a particular lot, the VIC must be immediately notified to assess the condition. Once the condition is identified/confirmed by the VIC, the disposition will be communicated to the rejecter(s) and the CFIA Evisceration Floor Inspector.
The licence holder may request CFIA veterinary inspector for comments on the condition found on form CFIA/ACIA 5639 "Poultry Rejection Process Control Evaluation Report".
7.8 Verification by CFIA
Role of CFIA veterinary inspector
The CFIA veterinary inspector is a highly trained professional whose professional judgement is to be considered by licence holder for every aspect of the Poultry Rejection Process.
Note
The CFIA veterinary inspector will maintain permanent presence during the evisceration operations.
Whenever a truck/lot is selected by the CFIA veterinary inspector for a Rejection Correlation Test, the rejecter must present each carcass removed from the evisceration line for suspect pathology to the veterinary inspector.
The licence holder will assist CFIA veterinary inspector in performing all the PRP activities as below:
- once per half shift:
- perform an RCT for unwarranted rejections (false positive) and carcasses passed in error (false negative) on a lot randomly selected by the CFIA veterinarian
- perform a detailed inspection on all carcasses from the selected lot which are removed from the evisceration line for suspected pathology (not for carcasses with only processing defects) after the rejecter has made a decision for each such carcass as previously described under Phase 2 of implementing the PRP
- verify that the rejection data collected and collated by the rejecter for the lot accurately represents the rejections reported on the operator's "Condemnation/Rejection Report for Poultry" (if issued by the operator) disposition report for that lot
- record and enter false positive and negative data and all of the other information listed into the Excel spreadsheet to generate the control charts and to track trends in corresponding charts and graphs
- review the control charts and graphs for false positive and negative to assess industry control over the rejection process
CFIA evisceration floor inspector
The CFIA Evisceration Floor Inspector will monitor the rejection process throughout evisceration operations whenever the CFIA veterinary inspector is not present within the evisceration area and will be performing following PRP duties:
- screen each carcass that was removed by the defect detectors for suspected generalized pathology and that was not rejected by the rejecter
- ensure that the carcass and viscera that are false negative, then licence holder must set the carcass and viscera at designated location for a detailed Veterinary inspection
- advise the CFIA VIC whenever anything suspicious or abnormal takes place such as:
- there appears to be unwarranted rejections
- the rejecter is replaced just before the veterinary inspector performs an RCT
- a new untrained industry employee starts to perform rejections while not being trained by the trainer
- lot with a high level of rejections or with an uncommon disease/condition is being eviscerated
- large number of viscera are being rejected by the viscera defect detector due to contamination and the cause is not corrected in a timely manner
8. Process control(s)
Introduction
"Process controls" is not specifically defined in the Safe Food for Canadians Act or in the Safe Food for Canadians Regulations.
Generally, "Process controls are a system of controls used by a business to prevent and control hazards at each step of the food production process in a predictable, stable and consistent manner."
Under MPIP, "Process Control (PC) is a control used at a point or step that will contribute to the effectiveness of the related CCP(s) or post mortem inspection activities".
Relationship of PC to CCP
The process control (PC) contributes to the effectiveness of an associated critical control point (CCP). Any deviation at a CCP will require an evaluation of the supporting PC(s) as part of the deviation procedures associated with that CCP. Therefore, the PC must also be linked to the CCP(s).
The current HACCP Generic Model Chicken (Poultry) Slaughter includes the following CCP's:
- giblet and neck Harvesting
- on-line final carcass trimming
- off-line reprocessing-reconditioning, salvaging
- chilling
Note
The licensed operator may have additional CCP's in addition to the above CCP's.
Relationship of the PC to the post-mortem process under MPIP
The PCs listed below must be utilized by MPIP poultry slaughter establishments to control the evisceration process:
- Evisceration standards (ES) – Section 8.1
- Presentation standards (PS) – Section 8.2
- Defect detection standards (DDS) – Section 8.3
- Carcass dressing standards (CDS) – Section 8.4
The PC's contribute to the effectiveness of the following activities:
- Viscera defect detection:
- Evisceration standards
- Presentation standards
- Cavity defect detection:
- Evisceration standards
- Presentation standards
- Final examination:
- Evisceration standards
- Presentation standards
- Defect detection standards (carcass group)
- Carcass dressing standards
- Carcass parts and giblet harvesting:
- Evisceration standards
- Defect detection standards
- Carcass dressing standards
- Salvaging:
- Evisceration standards
- Defect detection standards (carcass group, viscera group)
- Carcass dressing standards
- On-line reprocessing and reconditioning
- Evisceration standards
- Defect detection standards
- Off-line reprocessing and reconditioning
- Evisceration standards
- Defect detection standards
The written program for PCs must also contain operator specific information as specified within the "The Food Safety Enhancement Program approach to a preventive control plan".
Elements of process control:
The MPIP process controls are designed to keep process in control on an ongoing basis and gives opportunity to the licence holder to take corrective actions to bring the process back into control. A failed process control does not lead to automatic regulatory non-compliance. The licence holder is deemed to be under regulatory non-compliance after 3 failed tests.
Note
- Under certain situations the CFIA reserves the right to declare operator's process out of compliance.
- While the process control gives feedback on about the evisceration process, the final product produced should meet the regulatory compliance at all times.
Sampling procedure
Sample procedure for carcasses in this guidance document were developed using sampling plans indexed by the AQL. Following principles were used when designing sampling procedures:
- The defect detection process monitoring is performed using an acceptance sampling plan, the ISO 2859-1 sampling plan.
- The acceptance quality level (AQL) is the maximum percent defective (or the maximum number of defects per 100 units) that, for the purposes of sampling inspection, can be considered satisfactory as a process average (ISO 2859-1:1999).
- The acceptance number (Ac) is the largest number of defects permitted in a sample in order that the lot is accepted for a specific acceptance quality limit.
- A lot is the equivalent of one hour of production volume per evisceration line per shift.
- Sampling time to be randomly selected for each hour of production.
- A nonconformity is a poultry carcass with one or more processing or pathological defects as defined in this section.
- A defective carcass is a carcass with a defect that cannot be corrected on the evisceration line, for example, a carcass requiring disposition and with extensive processing defects.
- Other carcasses with defects that are not considered as nonconforming items under the present system are referred to as carcasses with on-line trimmable defects.
Monitoring procedures
Process control monitoring has three (3) general components:
- Process evaluation
- Corrective measure(s) evaluation
- Post-chill production verification
Process evaluation monitors the process in the normal state. It determines if the process meets the standards on an on-going basis. It is performed at a consistent frequency on successive lots. The process evaluation may use a statistical standard that does not exceed the established acceptance quality limit. For each evaluation which reaches or exceeds the rejection number the licence holder must inform CFIA personnel and must conduct an investigation to determine the probable cause to help decide on the best course of corrective action.
Corrective measure(s) evaluation is an assessment of the adequacy of corrective measures that have been implemented following a rejected sample. It determines if the licence holder's corrective actions are effective and can bring the process back into control. This corrective measure evaluation is to be conducted after the licence holder has undertaken corrective measures and the CFIA is informed (as CFIA will conduct a correlation test). A corrective measure(s) evaluation must be conducted within 10 minutes after implementing corrective action(s).
Post chill product verification is to be used to ensure that potentially defective product of rejected lot(s) produced during the process evaluation and corrective measure(s) evaluation meet the standard or should be held for rework.
Decision tree
The decision trees for PC's are to be used by the licence holder's to monitor the process controls and to ensure that the process is kept under control. These are presented in the document with each process control.
Once the MPIP Phase 3 commences, the switching rules must be utilized as per the decision tree. The switching rules alternates process between "process control evaluation" and "corrective measures evaluation".
Note
The licence holders must empower employees to take immediate action whenever they notice a potential loss of control of process.
CFIA's regulatory test procedures for assessing licence holder's PC controls
Certified CFIA veterinarians and inspectors will be responsible for verifying the operator's monitoring tests, corrective actions and records. Therefore, CFIA inspection staff will be performing correlation tests or independent tests (as deemed necessary by the veterinary inspector) to verify the company's compliance and performance.
Preference will be given by CFIA to the correlation tests.
CFIA staff will use CVS Task 1.5.12 to verify the operator meets the regulatory requirements for the MPIP. Additionally, CFIA staff will use CVS Task 1.5.06 to verify the establishment employees are performing the MPIP tests appropriately.
Correlation tests:
Correlation testing consists of the CFIA conducting an evaluation of a test being performed by the licence holder's monitor(s) according to the following parameters:
- Tests will be performed on each evisceration line at the frequency indicated for each process control. This frequency may be increased according to the licence holder's compliance to the monitoring procedures.
- Times for the correlation tests will be randomly selected prior to the start of the shift.
- The licence holder must examine the same carcasses and associated viscera at the same time as the CFIA inspection staff.
- The monitor will be evaluated by CFIA for the sampling method used, correct interpretation of defects, correct recording of defects, correct assessment of the process, correct application of the decision tree and the implementation of corrective actions (if necessary).
- The correlation test frequency may be increased by CFIA if there is non- compliance to the regulations and monitoring procedures.
- The CFIA may perform an additional correlation test at any time as a further assurance of process control or if they feel that standards are not being met for any reason.
- If the CFIA's evaluation demonstrates deficiency in the industry's process and/or the monitoring thereof, immediate corrective measure must be initiated by the licence holder.
- The VIC reserves judgement if the licence holder's written procedure is to be re-examined and needs to be amended accordingly.
- The test results of correlation tests or additional correlation tests will be recorded by CFIA on a separate log or on the licence holder's records such that CFIA tests can be distinguished from tests conducted by the licence holder's process control monitor.
- The Rejection correlation test (RCT) is performed on each truck/lot by the CFIA veterinary inspector which assesses the rejecter's competency under Poultry rejection process (PRP).
Independent tests:
In addition to the correlation tests, CFIA may perform independent process control tests at any time.
When CFIA performs independent verification tests on heavy carcasses (such as, turkey carcasses), the licence holder must ensure minimal manipulation during the carcass collection and provide assistance or adequate equipment.
If the CFIA's independent evaluation demonstrates a lack of compliance to the process control and/or the monitoring thereof:
- immediate corrective measures must be initiated by the licence holder
- the licence holder must request permission from CFIA for continuation of the evisceration process
- the VIC must be notified when the written procedure is reassessed and/or amended by the licence holder
Note
Effective control by the licence holder over the process control may lead to reduction of the verification frequency and corrective actions by inspection personnel (such as see presentation standards test process controls).
8.1 Evisceration standards (ES)
The evisceration standards is a PC to prevent/control contamination caused by accidents during venting, opening, evisceration operations, defect detection, carcass parts and giblet harvesting, salvage and reprocessing and reconditioning.
CFIA staff will use CVS Task 1.5.03 to verify the evisceration standards protocol meets all requirements.
ES process control monitoring has two (2) general components:
- Process evaluation
- Corrective measure(s) evaluation
The licence holder must empower its employees to take immediate action whenever they notice a potential loss of evisceration standards control.
Evisceration standards are used as a process control (PC) to contribute to the effectiveness of the related CCP(s) and post mortem examination activities.
Position for the on-line monitoring ES station
The monitoring for the ES must be performed on-line immediately follows the evisceration operations (including a back-up employee if required) and before the viscera and/or cavity defect detectors (including the handling of carcasses and/or viscera by other employees) in a manner to avoid sampling bias.
Evisceration standards (ES) test may be performed at the same station as the one used for monitoring Presentation standards (PS).
Evisceration standards station for tests, training and accreditation
On-line space (1 to 1.5 meters), prior to viscera defect detector(s) and to trimming the carcass or harvesting the viscera is required to perform the evisceration tests.
On-line space is also required for CFIA staff and licence holder's trainers to train and accredit the establishment trainers as cavity defect detectors. The space is also provided for the training and the accreditation of the carcass defect detectors by the accredit trainers. However, provisions for line space for evisceration standards and training/accreditation may be combined.
Longer space is required proportional to line speed and associated sampling procedures
ES defects
The evaluation of evisceration operations must include observing for the presence of either the following 2 defects within the carcass cavity and/or the cavity opening:
Faecal contamination
Any identifiable material determined to be from the lower gastrointestinal tract (intestines, caecum, cloaca).
Ingesta contamination
Any material determined to be from the upper gastrointestinal tract (crop, gizzard or proventriculus). Dry and localized ingesta covering an area of 2.5 cm2 (diameter of 1.8 cm) or a few isolated grains will not be counted as a defect.
Sampling procedure
The on-line testing procedure used for the ES must be similar to the random sampling selection specified for the Defect detection standards (DDS), as described in "Sampling Procedure". However, the carcass examination area is restricted to the cavity opening and the carcass cavity.
The following step-by-step sampling procedure has been developed to facilitate national uniformity and is designed to ensure that each carcass has an equal chance of being selected. Carcasses must be selected as described below to prevent sampling bias.
- Step 1. Randomly select a time for the test (minimum once per hour). At the selected time, begin the test by randomly identifying a carcass and picking the third subsequent carcass to be the first carcass in the sample.
- Step 2. Visually examine carcass cavity opening and carcass cavity.
- Step 3. Mentally count (add) the carcass, or use a mechanical counter for larger sample sizes.
- Step 4. Repeat steps 1 to 3 until the sample size has been reached.
- Step 5. Record all defects on the ES Defects Log.
Step 6. Determine if the sample indicates that the lot passed or failed. Take appropriate control action, if warranted, including those indicated by the ES Decision Tree.
Note
A carcass showing multiple defects must be scored as one defective carcass. When multiple defects are noted, record the defect which is most obvious.
Sampling frequency, sample size and acceptance criteria
An accredited employee of the licence holder must conduct scheduled tests on the specified number of carcasses on an hourly basis.
The sample size and the accept/reject numbers for the Evisceration Standards evaluation are indicated in the following table:
| Lot Size | Process evaluation | Corrective measure(s) evaluation | Chicken and Fowl | Turkey | ||
|---|---|---|---|---|---|---|
| AcTable Note 6 | ReTable Note 7 | AcTable Note 6 | ReTable Note 7 | |||
| ≤ 5,000 cphTable Note 5 (max.1 hour/lot) |
32 Carcasses (each hour) |
32 Carcasses (within 10 minutes)Table Note 8 |
3 | 4 | 5 | 6 |
| ≥ 5 001 cph (max.1 hour/lot) |
50 Carcasses (each hour) |
50 Carcasses (within 10 minutes)Table Note 8 |
5 | 6 | 8 | 9 |
Table Notes
- Table Note 5
-
cph = carcasses per hour
- Table Note 6
-
Ac = Accept number
- Table Note 7
-
Re = Reject number
- Table Note 8
-
Approximate delay required in order to evaluate the effect of the corrective measures at the evisceration standards station
Note
Effective control by the licence holder over the evisceration of carcasses must be done in a permanent and pro-active manner under the CFIA oversight.
For licence holders operating at a very low line speed, the licence holder may submit alternate sample size for examination by the VIC.
ES decision tree
Each element of the process control and their interaction are explained below and are presented in the decision tree.
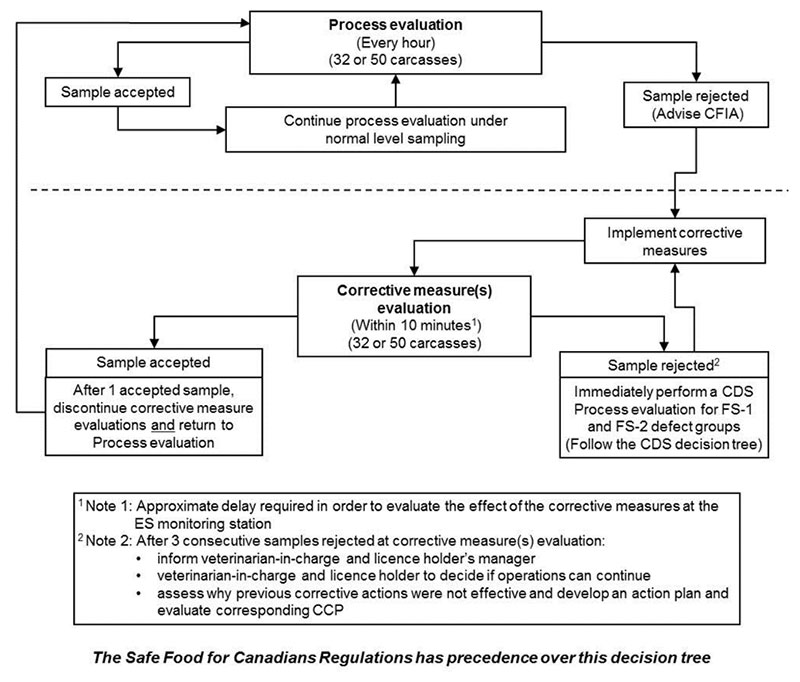
Description for Evisceration standards decision tree
Step 1: Process evaluation
With process evaluation under normal level sampling, done every hour for 32 or 50 carcasses, there are two possibilities:
- Sample accepted, which leads to continue process evaluation under normal level sampling, which returns you to the beginning of the process evaluation.
- Sample rejected: Advise the Canadian Food Inspection Agency and implement a corrective measure which leads to the second step, Corrective measure evaluation.
Step 2: Corrective measure evaluation
With corrective measure evaluation, within 10 minutes (See Note 1 below) for 32 or 50 carcasses, there are two possibilities:
- Sample accepted: After 1 accepted sample, discontinue Corrective measure evaluations and return to "Process evaluation"
- Sample rejected (see Note 2 below): Immediately perform a Carcass dressing standard process evaluation for defect groups Food Safety – 1 (faecal contamination) and Food Safety – 2 (ingesta contamination). Follow the Carcass dressing standard decision tree.
Note 1
Approximate delay required in order to evaluate the effect of the corrective measures at the ES monitoring station.
Note 2
After 3 consecutive samples rejected at corrective measure(s) evaluation:
- inform veterinarian-in-charge and licence holder's manager
- veterinarian-in-charge and licence holder to decide if operations can continue
- assess why previous corrective actions were not effective and develop an action plan and evaluate corresponding CCP
The Safe Food for Canadians Regulations has precedence over this decision tree
Recording ES defects
A separate ES Defects Log must be used for each species of poultry.
For abattoirs with more than one shift per day, test results for each shift must be considered independently because of differences of personnel and supervisors between shifts and test results for each shift must be considered independently. Thus, test results must be recorded on separate ES Defects Log.
Carcasses are scored as a defective sample unit for the presence of any distinguishable defect listed in section "ES Defects". A carcass showing multiple defects is scored as one defect (such as a carcass contaminated with fecal material and with ingesta is considered as one defective carcass).
Defects are scored, a total score is determined and acceptability is determined by comparing the score to the applicable acceptance and rejection numbers.
Process out of control – action to be taken
The licence holder has the responsibility to implement timely and effective corrective actions immediately following each evisceration standards test indicating that the number of evisceration defects has reached or exceeded the reject number. In each case, they must advise CFIA personnel and must conduct an investigation to determine the probable cause to help decide on the best course of corrective action.
Process evaluation
When the test result reaches the rejection number, it indicates that the process is under questionable control. Therefore, corrective measures must be implemented.
CFIA staff must be informed by the licence holder after each rejected sample.
Corrective measure(s) evaluation
Whenever a Process Evaluation is failed the licence holder must conduct an investigation to determine the cause and take effective corrective action. The efficiency of the corrective measures is evaluated by performing a Corrective Measure(s) evaluation. Corrective measures and additional corrective measure(s) evaluations are required until one (1) accepted sample demonstrates conformance to the standard. Effective preventative measures must be designed and implemented to prevent a reoccurrence as specified in the written PCP system of the licence holder.
If an Evaluation of the corrective measure(s) is rejected, implement corrective measures, initiate another corrective action and the affected product is verified by performing a CDS test on FS-1 (fecal material) and FS-2 (ingesta) ensuring that contaminated carcasses are not entering the chilling system. The decision tree for the CDS must be followed based on the results of the aforementioned tests.
Other considerations
The Safe Food for Canadians Regulations (SFCR) has precedence over the ES decision tree (corrective measures can be mandated at any time by the veterinary inspector).
Verification by the CFIA
When requested, the licence holder must assist the CFIA staff to ensure that the Evisceration standards (ES) process control have been implemented and are being performed according to the licence holder's written program.
CFIA will accomplish this by performing correlation tests with the licence holder's monitor as below:
- Process evaluation – When the licence holder is monitoring the ES, CFIA will perform a correlation test at a minimum of once per half shift/evisceration line.
- Corrective measure(s) evaluation – When the licence holder has implemented the corrective measures, the licence holder must conduct corrective measure evaluation test together as a correlation test with CFIA Inspector.
The CFIA may perform an additional correlation test at any time as a further assurance of process control or if they feel that standards are not being met for any reason.
| Test type | Process evaluation | Corrective measures evaluation |
|---|---|---|
| Monitoring by the industry monitor | Once per hour | Within 10 minutes after implementing corrective action(s) |
| Verification by CFIA staff | Once per half shift | At all times when corrective measures implemented are evaluated by licence holder. |
The Evisceration standard (ES) decision tree is to be used by the evisceration standards monitor and presented for reference by the CFIA.
8.2 Presentation standards (PS)
The presentation standards ensure adequate evisceration, so that on-line defect detectors can perform proper detection. Presentation standards aid accredited cavity and viscera defect detectors to carry out their responsibilities in compliance with defect detection standards by ensuring that carcasses and corresponding viscera are presented in a uniform and consistent manner. These standards are designed to ensure that evidence of disease is not lost (such as missing viscera), nor hidden (such as inadequate abdominal opening) during visual examination by the defect detectors.
PS process control monitoring has 2 general components:
- Process evaluation
- Corrective measure(s) evaluation
Presentation standards are used as a process control (PC) to contribute to the effectiveness of the related CCP(s) and post mortem examination activities.
The licence holder must empower its employees to take immediate action whenever they notice a potential loss of presentation standards control (such as excessive missing viscera).
Presentation standards are applicable to all types of evisceration procedures regardless of the technology used such as whether manual evisceration or automated evisceration equipment which leaves the viscera either attached or physically separated from the corresponding carcass.
Presentation monitoring tests are performed on each evisceration line at a presentation test station located after evisceration and prior to viscera detection. Carcasses correctly identified by the cavity defect detectors for removal by the helper/trimmer must not be included in the sampling for the presentation tests.
Position for the on-line presentation standards (PS) station
The monitoring for the PS must be performed on-line immediately follows the evisceration operations (including a back-up employee if required) and before the viscera and/or cavity defect detectors (including the handling of carcasses and/or viscera by other employees) in a manner to avoid sampling bias.
Presentation standards (PS) test may be performed at the same station as the one used for monitoring Evisceration standards (ES).
Presentation standards station for tests, training, and accreditation
On-line space (1 to 1.5 meters), prior to viscera defect detector(s) and to trimming the carcass or harvesting the viscera is required to perform the presentation tests.
On-line space is also required for CFIA staff and licence holder's trainers to train and accredit the establishment trainers as cavity defect detectors. The space is also provided for the training and the accreditation of the carcass defect detectors by the accredit trainers. However, provisions for line space for presentation and training/accreditation may be combined.
Longer space is required proportional to line speed and associated sampling procedures
PS defects
During presentation monitoring, the three (3) defects (No Viscera, Viscera Parts Missing, and Inadequate Abdominal Opening) must be counted as presentation errors. These defects are described in the following sub-section and included within the AQL for presentation tests:
- No viscera
- For chicken, turkey and quail – carcasses are presented without the viscera or the viscera are presented with the heart and liver missing.
- For fowl – the duodenum must be missing in addition to the liver and the heart.
- Viscera parts missing
- For chicken, turkey and quail – presentation of the viscera with over ½ of the heart or over ½ of the liver missing. There must be at least one intact lobe of the liver present for defect detection purposes.
- For fowl – presentation of the viscera with over ½ of the heart or over ½ of the liver missing or the duodenum missing, with a maximum of one defect per carcass. Multiply the total number of hearts missing by 0.1.
- For mature poultry (including chicken roasters) – missing spleens count as an error if a significant percentage are missing as determined by the VIC.
- If a missing heart is combined with a missing liver or a missing duodenum, a maximum of one defect is counted.
- Inadequate abdominal opening
- Presentation of carcasses with an inadequate abdominal opening makes it impossible to examine the abdominal cavity properly. This may result from pieces of skin or flesh obstructing the opening, the anus or cloaca that have remained attached, or any obstacle located in the incision hampering the presentation and view inside. The abdominal opening must be large enough to allow presentation and examination of the inside of the carcass.
- For chicken and light fowl, a cut made within 2 cm of the point of the keel is adequate.
- For turkey, heavy fowl and roasters, a cut made within 3 cm of the point of the keel is adequate.
- For quail, a cut made within 1.5 cm of the point of the keel having a minimum opening diameter of 2.8 cm is adequate.
- Mutilated carcasses having obstructions which interfere with examination of the cavity must also be scored as an "Inadequate Abdominal Opening".
- Unless specified otherwise, each of the above listed errors will receive a score of 1 with a maximum of 1 error allowed per carcass.
Other defects to be controlled by the PCP system
The following six defects are not to be included as errors as part of the presentation tests and are not included in the AQL for the presentation tests. In the case of repetitive occurrence, as determined by the licence holder or the VIC, they must be controlled as part of the licence holder's PCP system:
Unopened carcass
Carcass presented without any abdominal incision.
Viscera not removed from cavity
Carcass presented with an abdominal incision but viscera are not sufficiently drawn from the abdominal cavity to permit detection or inspection.
Carcass not hung by legs
Carcass hung by the neck or a wing.
Water pooled within the cavity
Accumulated water may mask evidence of pathological and/or processing defects (such as airsacculitis and faecal contamination).
Contaminated viscera
Severe contamination to the extent that evidence of pathology is obscured
(such as generalized airsacculitis).Hearts and livers not visible
Viscera portions to be examined are present, but hidden behind the gizzard on a consistent basis.
Carcasses with presentation defects (a) to (c) (unopened carcass, viscera not removed from cavity and carcass not hung by legs) must be removed from the line (before or by the helper/trimmer) for verification of cavity and viscera defects. They must be identified and kept separate from carcasses for salvaging and not be allowed to unduly accumulate on racks.
Errors must be corrected as quickly as possible, and the carcasses rehung on the line in order not to compromise product safety due to bacterial multiplication. If not, they must be condemned as "licence holder rejects".
Sampling procedure
The sampling must be done at constant intervals, such as, every fifth carcass, every third carcass. To avoid sample bias, randomly select a carcass by picking one, then count a predetermined number of carcasses, such as, third one, and then examine this carcass and corresponding viscera. This carcass must be the first one of the sample. Repeat the procedure for each subsequent carcass until the required number is examined.
The last step consists of checking the line speed.
The total of error incidences is the score for that examination station and eviscerating line.
Sampling frequency, sample size and acceptance criteria
Sampling frequencies
The frequency of licence holder monitoring and CFIA verification tests on each eviscerating line must be based on the licence holder's ability to maintain uniform carcass presentation. This frequency may be reduced when there is confidence in the licence holder's presentation control, or it must be increased when this confidence has been lost, according to the following table.
| Test type | Process evaluation | Corrective measures evaluation | |
|---|---|---|---|
| Normal (low frequency) | Normal (regular frequency) | ||
| Monitoring by the industry monitor | Once per half shift | Once per hour | Within 10 minutes after implementing corrective action(s) |
| Verification by CFIA staff | Once per shift | Once per half shift | At all times when corrective measures implemented are evaluated by licence holder. |
Note
Effective control by the licence holder over the presentation of carcasses and viscera must be done in a permanent and proactive manner under the CFIA oversight.
For licence holders operating at a very low line speed, the licence holder may submit alternate a sample size for approval to the veterinary inspector.
Sample size, acceptance and rejection criteria
The sample size and the applicable accept and reject numbers must be governed by the line speed range as shown in the following table.
| Line speed (cphTable Note 9) | Process evaluation | Corrective measure evaluation | ||||
|---|---|---|---|---|---|---|
| Sample size | AcTable Note 10 | ReTable Note 11 | Sample size | AcTable Note 11 | ReTable Note 11 | |
| ≤ 5,000 cph | 32 | 3 | 4Table Note 12 | 32 | 3 | 4Table Note 12 |
| ≥ 5 001 cph | 50 | 5 | 6Table Note 12 | 50 | 5 | 6Table Note 12 |
Table Notes
- Table Note 9
-
cph: carcasses per hour
- Table Note 10
-
Ac = Accept number
- Table Note 11
-
Re = Reject number
- Table Note 12
-
If the total of missing viscera reaches half of the rejection number, and/or if the total of missing heart, for turkey and quail, reaches the rejection number, the licence holder must seek advice from a veterinary inspector to determine if the defects are significant considering the pathology associated with the specific flock.
Presentation standard (PS) decision tree
Each element of the process control and their interaction are explained below and are presented in the decision tree.
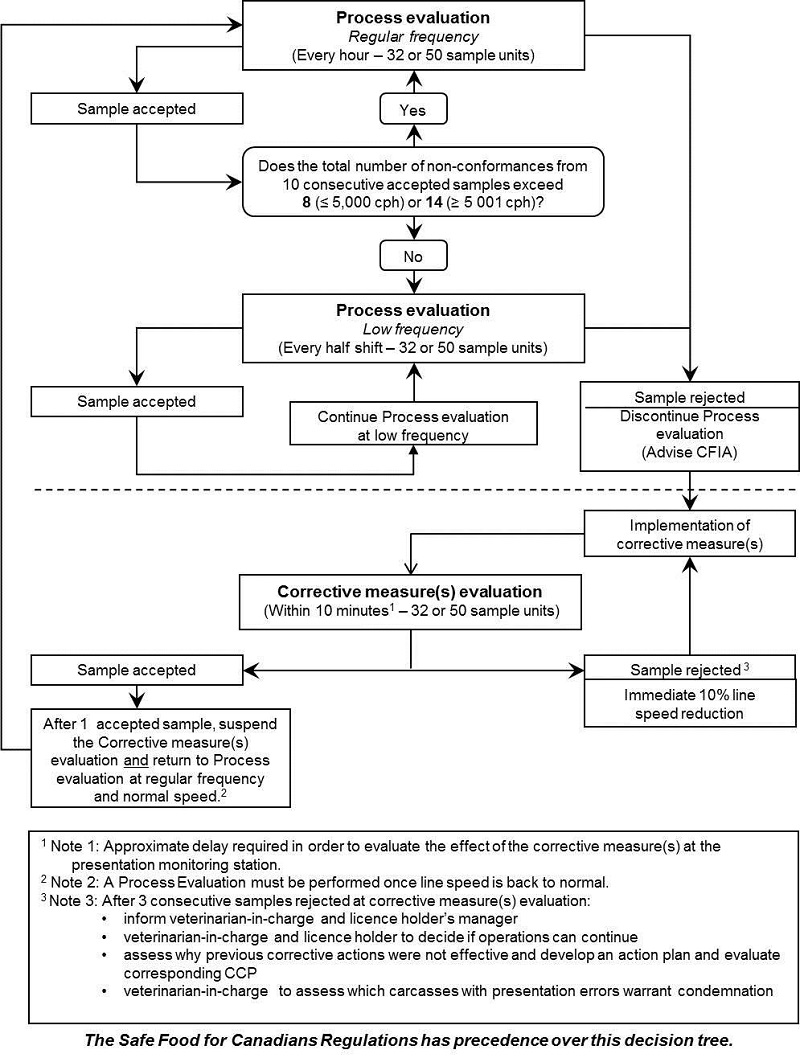
Description for Presentation standards decision tree
Step 1: Process evaluation
With Process evaluation (under Regular frequency), done every hour for 32 or 50 sample units, there are two possibilities:
- Sample accepted, which leads to continue process evaluation at Regular frequency, which returns you to the beginning of the Process evaluation.
- If the total number of non-conformances from 10 consecutive accepted samples under original inspection does not exceed 8 (≤ 5,000 carcasses per hour) or 14 (≥ 5 001 carcasses per hour), it leads you to go to Process evaluation under Low frequency
- If the total number of non-conformances from 10 consecutive accepted samples under original inspection does exceed 8 (≤ 5,000 carcasses per hour) or 14 (≥ 5 001 carcasses per hour), it leads you to go to Process evaluation under Regular frequency
- Sample rejected: Advise the Canadian Food Inspection Agency and discontinue the Process evaluation, which leads to implementation of corrective measures and the Corrective measure evaluation
With Process evaluation (under Low frequency), done every hour for 32 or 50 sample units, there are two possibilities:
- Sample accepted, which leads to continue Process evaluation under low frequency which returns you to the beginning of the Process evaluation.
- Sample rejected: Advise the Canadian Food Inspection Agency and discontinue the Process evaluation, which leads to implementation of corrective measures and the Corrective measure evaluation
Step 2: Corrective measure evaluation
With Corrective measure evaluation within 10 minutes (see Note 1) for 32 or 50 sample units, there are two possibilities:
- Sample accepted: After 1 accepted sample, stop corrective measure(s) evaluation. Return to Process evaluation at regular frequency and increase to normal line speed. An evaluation must be performed once the line speed is back to normal (see Note 2). or
- Sample rejected (see Note 3): Immediate 10% line speed reduction, which leads to an implementation of corrective measures and Corrective measure evaluation.
Note 1
Approximate delay required in order to evaluate the effect of the corrective measure(s) at the presentation monitoring station.
Note 2
A Process evaluation must be performed once line speed is back to normal.
Note 3
After 3 consecutive samples rejected at corrective measure(s) evaluation:
- inform veterinarian-in-charge and licence holder's manager
- veterinarian-in-charge and licence holder to decide if operations can continue
- assess why previous corrective actions were not effective and develop an action plan and evaluate corresponding CCP
- veterinarian-in-charge to assess which carcasses with presentation errors warrant condemnation
The Safe Food for Canadians Regulations has precedence over this decision tree
Recording PS defects and line speed
A separate PS Defects Log must be used for each species of poultry.
For abattoirs with more than one shift per day, test results for each shift must be considered independently because of differences of personnel and supervisors between shifts and test results for each shift must be considered independently. Thus, test results must be recorded on separate PS Defects Log.
Defects are scored, a total score is determined and acceptability is determined by comparing the score to the applicable acceptance and rejection numbers.
Line speed
The line speed must be recorded for all tests. There is no maximum line speed for MPIP provided operations remain in compliance with MPIP requirements. However, if the line speed is reduced as a corrective action, then exceeding the decreased line speed is counted as a defect. Furthermore, licence holder must immediately correct the line speed.
Process out of control – action to be taken
The licence holder has the responsibility to implement timely and effective corrective actions immediately following each presentation standards test indicating that the number of presentation defects has reached or exceeded the reject number. In each case, they must inform CFIA personnel and must conduct an investigation to determine the probable cause to help decide on the best course of corrective action.
Process evaluation
If the total of missing viscera, for all species, reaches half of the rejection number, or, if the total of missing heart, for turkey and quail, reaches the rejection number, the licence holder must seek advice from a veterinary inspector to determine if the defects are significant considering the pathology associated with the specific flock. In these instances, corrective actions are required to ensure proper detection of pathology.
CFIA staff must be informed by the licence holder after each rejected sample.
Corrective measure(s) evaluation
Corrective actions implemented by the licence holder may include one or more of the following:
- equipment adjustment and/or maintenance
- adding employees
- reducing the line speed (if extra time is required by the defect detectors to examine the carcass and/or viscera due to excessive presentation defects)
- changing the flock (such as if extra time is required for the birds to empty their intestines)
- temporarily suspend the hanging of live birds
If a line speed reduction is chosen as the corrective action by the licence holder or is enforced by CFIA staff, then retests must be done 10 minutes after a line speed decrease or increase.
Immediately following a failed corrective measure(s) evaluation, the presentation standards monitor must notify the CFIA personnel and the designated representative of the licence holder. If corrective action taken by the licence holder fails to reduce the following evaluations to an acceptable level, then the licence holder may re-assess the presentation status; implement additional corrective actions and a 10% line speed reduction.
After 1 accepted corrective measure(s) evaluation, not exceeding the acceptance number (Ac), the process is back to normal process evaluation since the evaluation determined that implemented corrective measures have been adequate and effective.
If 3 consecutive corrective measure(s) evaluation tests are rejected, the licence holder must:
- determine to what extent the deviations from the presentation standards affect the detection of defective carcasses and viscera
- evaluate and approve further corrective action(s)
- determine if live hanging operations may continue
- the licence holder must evaluate and assure the safety and wholesomeness of affected product and submit evaluation results to CFIA for examination by an veterinary inspector
- submit a written action plan to the CFIA (including amendments to current PCP system) which is designed to resolve and prevent a recurrence
Other considerations
In the case of recurring non-compliance, unusually high condemnation rates or consistently ineffective corrective action, the veterinary inspector must be kept informed of all test results for analysis and possible further action.
The Safe Food for Canadians Regulations (SFCR) has precedence over the PS decision tree (corrective measures can be mandated at any time by the veterinary inspector).
Verification by the CFIA
When requested, the licence holder must assist the CFIA staff to ensure that the Presentation standards (PS) process control have been implemented and are being performed according to the licence holder's written program.
CFIA will accomplish this by performing correlation tests with the licence holder's monitor as below:
- Process evaluation – When the licence holder is monitoring the PS at normal frequency, CFIA will perform a correlation test at a minimum of once per half shift per evisceration line.
CFIA will perform correlation test at a minimum of once per shift, when the licence holder qualifies for a reduced frequency, (as mentioned in the section explaining the licence holder's frequency).
- Corrective measure(s) evaluation – When the licence holder has implemented the corrective measures, the licence holder must conduct corrective measure evaluation test together as a correlation test with CFIA Inspector.
CFIA staff may perform an additional correlation at any time as a further assurance of process control or if they feel that standards are not being met for any reason.
The frequency of licence holder monitoring and CFIA verification tests on each eviscerating line must be based on the licence holder's ability to maintain uniform carcass presentation.
| Test type | Process evaluation | Corrective measures evaluation | |
|---|---|---|---|
| Normal (low frequency) | Normal (regular frequency) | ||
| Monitoring by the industry monitor | Once per half shift | Once per hour | Within 10 minutes after implementing corrective action(s) |
| Verification by CFIA staff | Once per shift | Once per half shift | At all times when corrective measures implemented are evaluated by licence holder. |
The Presentation Standard (PS) Decision tree is to be used by the presentation standards monitor and presented for reference by the CFIA.
8.3 Defect detection standards (DDS)
The defect detection standards establish the criteria for verifying the licence holder's performance for defect detection. DDS are designed to include defect detection for the carcass exterior, cavity and viscera. A separate AQL is assigned to carcass defects, to cavity defects and to viscera defects. All 3 AQLs, including a zero tolerance for septicaemia / toxaemia applies to all abattoirs operating under the MPIP.
DDS process control monitoring has 3 general components:
- Process evaluation
- Corrective measure(s) evaluation
- Post-chill production verification
The licence holder must empower its employees to take immediate action whenever they notice a potential loss of Defect detection standards.
DDS are used as a process control (PC) to contribute to the effectiveness of the related CCP(s) and post mortem examination activities.
DDS have been established in 2 versions. The difference between the versions is the addition or exclusion of the internal cavity defects evaluation.
- "Version 1" applies to a licence holder that does not use an approved on-line reprocessing and reconditioning process (or during validation). The monitoring defects have been divided into 3 groups: carcass defect group, viscera defect group and cavity defect group (including Septicaemia /Toxaemia).
- "Version 2" applies to an licence holder that does use an approved on-line reprocessing and reconditioning process (or during validation). Under this version, the cavity defects are removed such that 2 defect groups are to be evaluated: carcass defect group and viscera defect group (including septicaemia, toxaemia).
National AQLs and monitoring defects lists
The National AQLs were determined based on prevalence data resulting from national surveys in slaughtering establishments.
| Defect groups | Chicken | Fowl | Turkey | Quail |
|---|---|---|---|---|
| Carcass defects group | 0.4% | 0.4% | 0.4% | 0.4% |
| Viscera defects group | 0.4% | 0.4% | 0.4% | 0.4% |
| Cavity defects group | 1.5% | 1.5% | 1.5% | 1.5% |
Note
There is a "zero" tolerance for the septicaemia, toxaemia defect in the carcass and viscera defect groups.
Position for the on-line monitoring DDS station
- "Version 1": downstream from the defect detection with team of establishment carcass/viscera/cavity detectors
- "Version 2": downstream from the defect detection with team of establishment carcass/viscera defect detectors; and when cavity defect detectors are positioned after the DDS station
- before or after the helper/trimmer
- before viscera is harvested (or discarded) or the carcass is trimmed (other than by the helper/trimmer) and before the internal cavity is vacuumed
The licence holder must choose version 1 or version 2 for each evisceration line.
Defect detection standards (DDS) station
On-line space is required, proportional to the line speed (1 to 2 meters), after viscera detection, but prior to harvesting the viscera.
Carcasses must be synchronized to their corresponding viscera with both readily accessible to the inspector throughout the length of the inspection station.
These line space requirements may be combined with the CFIA on-line station required to meet export requirements.
DDS defects
The following defects must be counted during defect detection monitoring.
| Carcass defect group (versions 1 and 2) | Chicken | Fowl | Turkey | Quail |
|---|---|---|---|---|
| Ascites | X | X | X | X |
| Cellulitis (NTOLTable Note 13) and peri-cloacal cellulitisTable Note 14 | X | X | X | X |
| Dark coloured carcasses | X | X | X | X |
| Emaciation with extreme thinness | X | X | X | X |
| Inadequate bleeding (bright red carcass) | X | X | X | X |
| Pendulous crop with emaciation | X | X | X | |
| Septicaemia, toxaemia | X | X | X | X |
| Sternal bursitis, infected breast blister (NTTable Note 15) | X | X | X | |
| Xanthomatosis | X | |||
| Others: Arthritis, synovitis and valgus varus deformity with emaciation | X | X | X | X |
Table Notes
- Table Note 13
-
NTOL: Not trimmable on-line (too extensive for trimming at normal line speed)
- Table Note 14
-
Peri-cloacal cellulitis in considered to be a defect only for chicken under version 1 only
- Table Note 15
-
NT: Not trimmable (too extensive for trimming)
| Viscera defect group (versions 1 and 2) | Chicken | Fowl | Turkey | Quail |
|---|---|---|---|---|
| Adenocarcinoma | X | |||
| Airsacculitis | X | X | X | X |
| Contamination such as faecal, bile, ingesta, extraneous material | X | X | X | X |
| Emaciation as visible on heart, gizzard | X | X | X | X |
| Hepatitis | X | X | X | X |
| Lymphoid leukosis | X | |||
| Marek's disease – visceral form | X | X | ||
| Peritonitis | X | X | X | X |
| Septicaemia, toxaemia | X | X | X | X |
| Other conditions such as osteomyelitis, tumors | X | X | X | X |
| Cavity defect group (Version 1 only) | Chicken | Fowl | Turkey | Quail |
|---|---|---|---|---|
| Airsacculitis | X | X | X | X |
| Contamination such as faecal, bile, ingesta, extraneous material, intestine/cloaca attached | X | X | X | X |
| Peri-cloacal cellulitis | X | X | X | |
| Salpingitis | X | X | X | X |
| Other conditions such as, odour, tumours, granuloma in quail | X | X | X | X |
Special procedures for septicaemia, toxaemia
A carcass and corresponding viscera exhibiting signs of potential Septicaemia / toxaemia, as defined under the "Definitions of carcass defects" or "Definitions of viscera defects" sections is to be removed for a CFIA veterinary inspection. If the veterinary inspector suspects a septicaemia, toxaemia condition, the following procedures must be initiated:
- the licence holder must immediately notify all defect detectors that potential Septicaemia / toxaemia is found during a DDS evaluation
- "Corrective measure(s) evaluation" procedures and the "Post-chill product verification" for both the viscera and the carcass groups are performed as per the DDS decision tree
- the carcass and viscera are sent for a laboratory analysis confirmation
- if the laboratory analysis confirms the Septicaemia / toxaemia condition, the licence holder must initiate corrective measure(s) satisfactory to the veterinarian-in-charge
Sampling procedure
Note
When CFIA inspector is present on-line performing export inspection duties, DDS sampling must be conducted to reflect the performance of the defect detectors without being influenced by the on-line CFIA inspector(s).
The sampling can be performed separately for each defect group as long as the required sample size is met for each defect group. All defect groups are to be evaluated using the same carcass sample.
Each sampled carcass must be fully examined (the carcass exterior, the corresponding viscera and the carcass cavity for Version 1). The following step-by-step sampling procedure has been developed to facilitate national uniformity and is designed to ensure that each carcass has an equal chance of being selected. Carcasses must be selected as described below to prevent sampling bias.
- Randomly select a time for the test (minimum once per hour). At the selected time, begin the test by randomly identifying a carcass and picking the third subsequent carcass to be the first carcass in the sample. If the carcass lacks corresponding viscera, then pick the next complete set of carcass and viscera.
- Visually examine the carcass exterior, the viscera (heart and liver for young chickens and turkeys plus intestines and spleen for mature poultry) and the carcass cavity (under Version 2, the cavity examination is not required). The order of the examination is at the discretion of the monitor to permit the most efficient inspection possible consistent with the presentation of the carcass and corresponding viscera.
- If a missed defect is suspected, immediately remove the carcass and if applicable, the corresponding viscera, and hang it/them back on the rack provided.
- Mentally count (add) the carcass, or use a mechanical counter for larger sample sizes (such as, 125 carcasses).
- Repeat steps 1 to 4 until the sample size has been reached.
- After completing the on-line examination of the carcasses comprising the sample, carefully examine each hung-back carcass (and its viscera) and determine if it is defective. Record all defects in DDS Defects Log.
- Determine if the sample indicates that the lot passed or failed. Take appropriate control action, if warranted, including those indicated by the DDS Decision tree.
- Release removed carcasses for disposition or correction by a designated establishment employee or for the return of normal carcasses to the evisceration line.
Note
A carcass showing multiple defects must be scored as one defective carcass. Similarly, viscera showing multiple defects will be counted as one defect. When multiple defects are noted, record the defect which is most obvious.
Sampling frequency, sample size and acceptance criteria
Testing frequency and sample size
The DDS monitoring tests are based on a lot-by-lot evaluation determined to be an hour's production. Therefore, accredited plant employees must conduct scheduled randomized tests, once every hour of production, on a specified number of carcasses and corresponding viscera at the on-line station. Times for the tests must be randomly selected prior to the start of the shift. The process evaluation must always remain on a production lot of an hour.
| Lot Size | Process Evaluation | Corrective Measure(s) Evaluation | Post-chill Product Verification |
|---|---|---|---|
| (1 hour/lot) < 5,000 cphTable Note 16 | 32 Carcasses (every hour) | 32 Carcasses (within 10 minutes)Table Note 17 | 32 Carcasses (every 15 minutes) |
| (1 hour/lot) ≥ 5,001 cphTable Note 16 | 125 Carcasses (every hour) | 125 Carcasses (within 10 minutes)Table Note 17 | 32 Carcasses (every 15 minutes) |
Table Notes
- Table Note 16
-
cph = carcasses per hour
- Table Note 17
-
Approximate delay required in order to evaluate the effect of the corrective measures at the DDS station.
Acceptance and Rejection Numbers
| Line speed | Sampling Mode | Process and Corrective Measure(s) Evaluations | Postchill Product Verification (32 carcasses only) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AQLTable Note 18 0.4% (carcass and viscera groups) | AQL 1.5% (cavity group) | AQL 0.4% (carcass and viscera groups) | AQL 1.5% (cavity group) | ||||||
| AcTable Note 19 | ReTable Note 20 | AcTable Note 19 | ReTable Note 20 | AcTable Note 19 | ReTable Note 20 | AcTable Note 19 | ReTable Note 20 | ||
| ≤ 5,000 cphTable Note 21 | Normal (32 carcasses) | 0 | 1 | 1 | 2 | 0 | 1 | 1 | 2 |
| ≥ 5,001 cphTable Note 21 | Normal (125 carcasses) | 1 | 2 | 5 | 6 | ||||
Table Notes
- Table Note 18
-
AQL: Acceptance Quality Limit
- Table Note 19
-
Ac: accept
- Table Note 20
-
Re: reject
- Table Note 21
-
cph: carcasses per hour
Note
Septicaemia, toxaemia has a "zero" tolerance.
Defect detection standards (DDS) decision tree
Each element of the process control and their interaction are explained below and are presented in the decision tree.
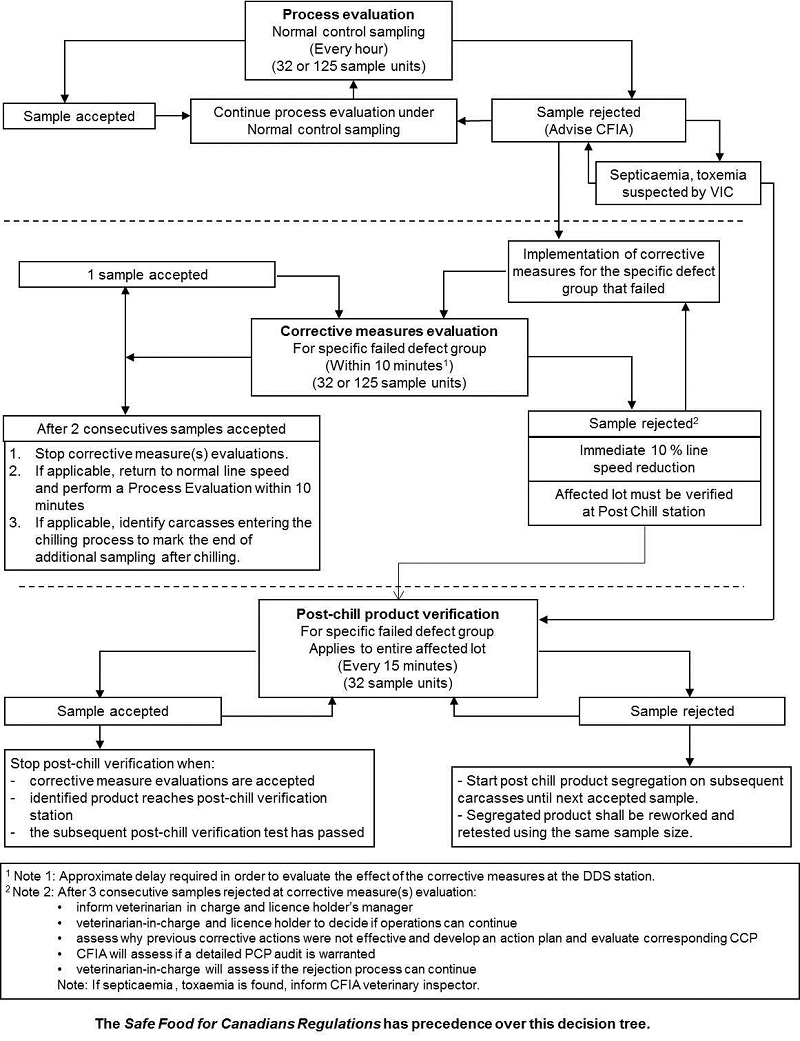
Description for Defect detection standards decision tree
Step 1: Process evaluation
With Process Evaluation under Normal Control Sampling, done every hour for 32 or 125 sample units, there are two possibilities:
- Sample accepted, which leads to continue Process evaluation under Normal Control Sampling, which returns you to the beginning of the Process Evaluation.
- Sample rejected: Advise the Canadian Food Inspection Agency (if Septicaemia / toxaemia are suspected by the veterinarian in charge, implementation of corrective measures for specific defect group, which leads to the second step, Corrective measure evaluation and go to the third step, Postchill product evaluation), which can lead to:
- Continue Process evaluation under normal control sampling, which returns you to the beginning, or
- Implementation of corrective measures for specific defect group, which leads to the second step, Corrective measure evaluation
Step 2: Corrective measure evaluation
With Corrective measure evaluation for a specific defective defect group, within 10 minutes (see Note 1) for 32 or 125 sample units, there are two possibilities:
Sample accepted: After 2 consecutive samples accepted,
- Stop corrective measure(s) evaluations.
- If applicable, return to normal line speed and perform a Process evaluation within 10 minutes
- If applicable, identify carcasses entering the chilling process to mark the end of additional sampling after chilling.
or
- Sample rejected (see Note 2): Immediate 10% line speed reduction and the affected lot must be verified at the post chill station, which leads to the third step, Postchill product verification or there is an implementation of corrective measures for a specific defect group, which returns you to Corrective measure evaluation
Step 3: Post-chill product verification
With Post-chill product verification for a specific defective defect group and applies on entire affected lot (Every 15 minutes for 32 sample units), which can lead to:
- Sample accepted: Stop post-chill verification when
- corrective measure evaluations are accepted;
- identified product reaches post-chill verification station; and
- the subsequent post-chill verification test has passed
or
- Sample rejected:
- Start post chill product segregation on subsequent carcasses until next accepted sample.
- Segregated product shall be reworked and retested using the same sample size.
Note 1
Approximate delay required in order to evaluate the effect of the corrective measures at the DDS station.
Note 2
After 3 consecutive samples rejected at corrective measure(s) evaluation:
- inform Veterinarian in Charge and licence holder's manager
- veterinarian-in-charge and licence holder to decide if operations can continue
- assess why previous corrective actions were not effective and develop an action plan and evaluate corresponding CCP
- CFIA will assess if a detailed PCP audit is warranted
- veterinarian-in-charge will assess if the rejection process can continue
Note
If septicaemia, toxaemia is found, inform CFIA veterinary inspector.
The Safe Food for Canadians Regulations has precedence over this decision tree.
Recording DDS defects
A separate DDS Defects Log must be used for each species of poultry.
For abattoirs with more than one shift per day, test results for each shift must be considered independently because of differences of personnel and supervisors between shifts and test results for each shift must be considered independently. Thus, test results must be recorded on separate DDS Defects Log.
Carcasses or viscera are scored as a defective sample unit for the presence of any distinguishable defect listed in section, "National AQLs and monitoring defects lists" of this document. A carcass or viscera showing multiple defects under the same defect group is scored as one defect (such as a carcass with inadequate bleeding and emaciation = one defective carcass).
A carcass with defects under different defect groups is scored as one defective carcass for each group (such as a carcass with ascites and hepatitis would = one defect in the Carcass Defect Group and one defect in the Viscera Defect Group).
Defects are scored in their respective defect group, a total score for each group is determined and acceptability is determined by comparing the score to the applicable acceptance and rejection numbers for that group.
Process out of control – action to be taken
Process evaluation
During the process evaluation, each defect group is to be monitored independently of the other defect groups, using the same sample.
When the test result reaches the rejection number for a specific defect group, it indicates that the process is under questionable control. Therefore, corrective measures must be implemented for this specific defect group and additional on-line testing is required.
Note
Scheduled randomized hourly pre-chill process evaluation tests must continue independently from corrective measure(s) evaluation tests and post chill product verification tests.
CFIA staff must be informed by the licence holder after each rejected sample.
Corrective measure(s) evaluation
Once corrective measures have been implemented, the efficacy of these measures must be evaluated and the evaluation must only apply to the defect group under questionable control. Corrective measures and additional corrective measure(s) evaluations are required until 2 accepted samples demonstrate conformance to the standard.
When a corrective measure(s) evaluation is rejected, the licence holder must immediately reduce the line speed by 10% and implement corrective measure(s). The efficacy of those corrective measures will be evaluated within 10 minutes.
Immediate post-chill product verification and potential product rework is required when 1 corrective measure(s) evaluation has been rejected. The verification is required only for the specific defect group(s). The end of additional sampling at post-chill verification station occurs once corrective measures have been accepted (2 accepted corrective measure(s) evaluations), by marking identified carcasses entering the chilling process.
Post-chill product verification
The post-chill verification must cover the entire amount of production or lot that was determined to be out of compliance at the pre-chill station (corrective measure(s) evaluation). If a Post-chill product verification sample is rejected, the establishment's monitor must then identify the affected product so that it may be segregated and accumulated for rework.
Other considerations
The licence holder must develop a written program that will clearly indicate how plant personnel will identify lots of carcasses requiring rework, isolate these lots, rework the carcasses, and verify that the rework is satisfactory.
Once a specified lot has been reworked for the appropriate defects, a rework verification test must be performed using the appropriate pass and fail criteria for that specific defect group. An accepted sample will result in releasing the retained lot.
If the first shift ends in corrective measure(s) mode and/or post-chill product verification mode, the second shift must continue with only the post-chill product verification mode until the end of affected carcasses.
If the last shift of the day (single or multiple shift establishments) ends in corrective measure(s) mode and/or post-chill product verification mode, the post-chill product verification mode must continue until the chilling system is emptied.
The Safe Food for Canadians Regulations (SFCR) has precedence over the DDS decision tree (corrective measures can be mandated at any time by the veterinary inspector).
Verification by the CFIA
When requested, the licence holder must assist the CFIA staff to ensure that the Defect Detection Standards (DDS) process control have been implemented and are being performed according to the licence holder's written program.
The CFIA will accomplish this by performing correlation tests with the licence holder's monitor as below:
- Process Evaluation – When the licence holder is monitoring the DDS, the CFIA will perform a correlation test at a minimum of once per half shift/evisceration line.
- Corrective Measure(s) evaluation – When the licence holder has implemented the corrective measures, the licence holder must conduct corrective measure evaluation test together as a correlation test with a CFIA Inspector.
CFIA staff may perform an additional correlation at any time as a further assurance of process control or if they feel that standards are not being met for any reason.
| Test type | Process evaluation | Corrective measures evaluation |
|---|---|---|
| Monitoring by the industry monitor | Once per hour | Within 10 minutes after implementing corrective action(s) |
| Verification by CFIA staff | Once per half shift | At all times when corrective measures implemented are evaluated by licence holder. |
The Defect detection standards (DDS) decision tree is to be used by the defect detection standards monitor and presented for reference by the CFIA.
8.4 Carcass dressing standards (CDS)
The licence holder is responsible to process chilled carcasses that have been dressed, trimmed and processed, under the minimum requirements specified under the CDS.
Carcass dressing standards (CDS) is an objective tool designed to ensure that the procedures used in preparing and approving a dressed food animal carcass are in control and that the product is produced in conformance with Canadian regulatory standards. These standards specify the operational requirements for dressing, trimming and processing of dressed approved carcasses.
CDS tests are performed on sample sets of dressed carcasses randomly selected throughout the production shift to validate the licence holder's performance in meeting prescribed product standards.
The CDS monitoring tool has 3 general components:
- Process evaluation
- Corrective measure(s) evaluation
- Post-chill product verification
The licence holder must empower its employees to take immediate action whenever they notice a potential loss of Carcass Dressing Standards.
CDS are used as a process control (PC) to contribute to the effectiveness of the related CCP(s) and post mortem examination activities.
When a licence holder chooses the CDS location and CDS monitoring (all or parts of the CDS test) as part of the final CCP, the licence holder should perform corrective measures for both MPIP and PCP (food safety).
CDS process control – ISO based test
Random sampling for CDS testing is performed by the licence holder using an "Acceptance Sampling Plan", the ISO 2859-1, Special Inspection Level S-3.
CFIA staff will use CVS Task 1.5.06 to verify that the operator meets the regulatory requirements for dressing poultry carcasses.
Position of the off-line CDS station
All defects must be monitored after dressing, trimming, pathology and processing and immediately prior to chilling.
CDS station
The licence holder must provide adequate facilities to hold and to examine sampled carcasses off-line prior to chilling.
The CDS station is an off-line monitoring station which must have safe access to pre-chill lines and be protected from traffic and obstructions. An easily cleanable rack with shackles must be provided to hold part of or the entire carcass sample and a table for examination. The station must include a clip board holder. The monitoring station must be under lighting that meets the minimum 2000 lux requirement.
Defects
The CDS defects are arranged by:
- 2 defect categories
- 7 defect groups
- defects (variable under defect group)
- The defects to be monitored under the CDS have been divided into 2 separate categories.
- the food safety (FS) category is designed to monitor the output of the dressing and evisceration procedures that may become a food safety risk
- the other, the dressing condition (DC) category which monitors the establishment's ability to remove unsuitable dressing conditions
- Each category has been divided into several defect groups that are to be monitored independently from the other defect groups. A defect group may also include various defects
- the food safety category has 3 different defect groups
- FS-1
- FS-2
- FS-3
- the dressing condition category has 4 different defect groups
- DC-1
- DC-2
- DC-3
- DC-4
- the food safety category has 3 different defect groups
- Each of the seven defect groups is evaluated using the same 8, 13 or 20 carcass sample.
CDS defects are defined as per the following tables:
| Defect group | Defect | Definition |
|---|---|---|
| FS-1 | Faecal material | Any identifiable stain and/or material determined to be from the lower gastrointestinal tract. |
| FS-2 | Ingesta (aggregate) | Identifiable stain and/or dry particles and/or liquid (aggregate) covering a minimum area > 5 mm (internal and external). |
| FS-3 | Gastro-intestinal Tract – lower GIT | Any combination of the following parts > 5 mm: intestine, caecum, cloaca (with mucosa tissue). |
| Gastro-intestinal Tract – upper GIT | Any combination of the following organs > 5 mm: oesophagus, crop, proventriculus and gizzard. |
| Defect group | Defect | Definition |
|---|---|---|
| DC-1 | Pathology – Airsacculitis | Identifiable lesions defined to be caseum or exudates or fibrin within the airsacs or in the thoracic and/or abdominal cavities measuring > 3 mm for chicken, fowl and quail and > 5 mm for turkeys. |
|
Pathology – Granuloma (Quail only) |
Yellowish granules of various sizes (1 mm to 15 mm) located in the air sacs or attached to abdominal organs. | |
| Pathology – Salpingitis | Inflammation, presence of liquid or solid material within the salpinx. | |
| Pathology – Cellulitis | Identifiable cellulitis lesions affecting underlying tissue of any size (normally yellow coloured skin). | |
| Pathology – Cutaneous Marek's Disease (Chicken only) | Enlarged feather follicles often with yellowish coloured surrounding skin covering an area of any size. | |
| Pathology – Keratoacanthoma (Chicken only) | Identifiable number of deep crater-shaped ulcers. | |
| Pathology – Synovitis/Tenosynovitis/Arthritis |
Inflamed leg joint or tendon (yellow and green coloured skin with or without subcutaneous œdema). Presence of liquid and solid material within the joint (Arthritis in Turkey and Quail). |
|
| DC-2 | Lungs |
Any lung portion measuring:
|
| Oil gland | Whole gland or fragment of an oil gland > 5 mm. | |
| Bruise |
Blood clumps or clots in the superficial subcutaneous tissue that cannot be washed out after slitting and the bruise extends into the deeper layers covering a minimum area:
Do not count as a bruise if also associated with a compound fracture. |
|
| DC-3 | Long shank |
The complete tibio-tarsal joint (both condyles) is covered to the point where the cartilaginous tissue becomes the bone. As a guideline:
Note: The cartilaginous portion may vary depending on the size of the carcass. |
| Trachea | Identifiable trachea portion > 5 mm. | |
| Breast Blister (fowl, turkey and quail only) |
Untrimmed or partially trimmed nodule on the keel bone (yellow/red/green material) in an area measuring:
|
|
| Mutilation and compound fracture |
Lacerated muscle and skin caused by equipment/procedures occurring in areas prior to the evisceration room covering a minimum area measuring:
Skinned elbows (bucked wings) without dislocation and trimmed portions (smooth cut) do not require trimming. Bone fracture (for example, leg or wing, but not wing tip) that has caused an opening through the skin. |
|
| Scabs or inflammatory tissue |
Aggregate scabs covering an area measuring a minimum of:
or inflamed tissue including Dorsal Myopathy measuring:
Do not consider scars or healed tissue. |
|
| Foreign material | Identifiable material such as grease and dust > 5 mm or any other foreign material > 2 mm (internal and external). | |
| Bile or ruptured yolk | Identifiable stain of bile or ruptured yolk sac > 5 mm (internal and external). | |
| DC-4 | Feathers and pinfeathers |
A minimum of:
For all poultry: a minimum of 1 feather ≥ 25 mm. |
Sample procedure
CDS process control – ISO based test
Random sampling for CDS testing is performed by the licence holder using an "Acceptance Sampling Plan", the ISO 2859-1, and Special Inspection Level S-3.
CDS test procedure
All carcass samples must be randomly selected from the evisceration line using a standard random selection technique to prevent sampling bias and must be included in the licence holder's written program. In order to correctly evaluate the defects in a consistent basis, it has been determined that the sampling, the examination and the recording of 20 carcasses sample should be completed in 7 to 10 minutes. The sampling procedure must be fully described in the company's written program and must be approved by the veterinary inspector.
To facilitate the application of random sampling, the following method is recommended:
- Step 1. Randomly select a time for the test.
- Step 2. At the selected time, begin the accumulation of the sample by identifying a carcass on the line, removing the third subsequent carcass from the line. This will be the first carcass in the sample. Hang the carcass on the provided shackle/rack.
- Step 3. Repeat Step 2 until the sample size has been reached.
- Step 4. Visually examine all carcasses (exterior, cavity and inside neck area). Remove the defect(s) from the carcass and hold the accumulated defect(s) for further recording.
- Step 5. After completing the examination of all the carcasses comprising the sample, record the defects on CDS Defects Log.
- Step 6. Determine if the sample indicates that the lot passed or failed considering each defect or group of defects individually. If applicable, take appropriate corrective measures as per the "CDS Decision Tree".
For more details on random sampling, see the CFIA designed Training Modules.
Sampling Frequency, Sample Size and Acceptance Criteria
Sample Size
The sample size for the Process evaluation (PE), Corrective measure(s) evaluation (CME) and Post-chill product verification (PPV) using the ISO 2859-1 sampling plan is based on the volume of production and the Special Inspection Level S-3.
| Lot size (PE & CME) | Process evaluation | Corrective measure(s) verification |
|---|---|---|
| ≤ 5,000 cphTable Note 22 | 13 carcasses (every hour) | 13 carcasses (within 10 minutes) |
| ≥ 5,001 cph | 20 carcasses (every hour) | 20 carcasses (within 10 minutes) |
Table Notes
- Table Note 22
-
cph = carcasses per hour
| Lot size (PPV) | Post-chill product verification |
|---|---|
| ≤ 5,000 cphTable Note 23 | 8 carcasses (every 15 minutes) |
| ≥ 5,001 cph | 13 carcasses (every 15 minutes) |
Table Notes
- Table Note 23
-
cph = carcasses per hour
Testing Frequency
The CDS monitoring tests are based on a lot by lot evaluation determined to be an hour's production. Therefore, an accredited plant employee must conduct scheduled randomized tests, once every hour of production, on a specified number of carcasses at the off-line station. Times for the tests must be randomly selected prior to the start of the shift.
The process evaluation must always remain on a production lot of 1 hour.
| Process evaluation | Corrective measure(s) evaluation | Post-chill product verification |
|---|---|---|
| Every hour | Within 10 minutesTable Note 24 | Every 15 minutes |
Table Notes
- Table Note 24
-
Approximate delay required in order to evaluate the effectiveness of the corrective measures at the CDS station.
CDS defect group – Accept (Ac) and Reject (Re) numbers
National Ac and Re numbers have been selected using the expected percentage of defective carcasses per defect group determined from a national survey for both Chicken and Turkey. The Ac and Re numbers were determined calculating an overall sampling rejection rate of a maximum of 5%. The process is considered to be under control when the test result does not exceed the acceptance number as shown in the following table.
Ac and Re numbers for process evaluation and corrective measure(s) evaluation
The following table contains the values to be used for Process evaluation testing and corrective measure(s) evaluation testing.
| Groups of CDS defects | Conditions | ≤ 5,000 cphTable Note 25 (13 carcasses) | ≥ 5,001 cph (20 carcasses) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chicken/Quail | Turkey | Fowl | Chicken/Quail | Turkey | Fowl | ||||||||
| Ac | Re | Ac | Re | Ac | Re | Ac | Re | Ac | Re | Ac | Re | ||
| FS-1Table Note 26 | Fecal material | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| FS-2 | Ingesta | 2 | 3 | 2 | 3 | 1 | 2 | 3 | 4 | 3 | 4 | 2 | 3 |
| FS-3 | Gastro-intestinal tract | 2 | 3 | 2 | 3 | 3 | 4 | 2 | 3 | 2 | 3 | 5 | 6 |
| DC-1Table Note 27 | Localized pathology | 0 | 1 | 1 | 2 | 0 | 1 | 1 | 2 | 1 | 2 | 1 | 2 |
| DC-2 | Lungs; oil gland; bruises | 6 | 7 | 7 | 8 | 7 | 8 | 9 | 10 | 10 | 11 | 10 | 11 |
| DC-3 | Long shank; trachea; breast blister; mutilation; compound fracture; scabs; inflamed tissue; foreign material; bile; ruptured egg yolk | 3 | 4 | 5 | 6 | 3 | 4 | 5 | 6 | 6 | 7 | 5 | 6 |
| DC-4 | Feathers | 5 | 6 | 6 | 7 | 6 | 7 | 8 | 9 | 9 | 10 | 9 | 10 |
Table Notes
- Table Note 25
-
cph = carcasses per hour
- Table Note 26
-
FS = Food safety;
- Table Note 27
-
DC = Dressing Condition
Ac and Re numbers for post-chill product Ac and Re numbers for post-chill product verification
This table contains the values to be used for post-chill product verification.
| Groups of CDS Defects | Conditions | ≤ 5,000 cphTable Note 28 (8 carcasses) | ≥ 5,001 cph (13 carcasses) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chicken/Quail | Turkey | Fowl | Chicken/Quail | Turkey | Fowl | ||||||||
| Ac | Re | Ac | Re | Ac | Re | Ac | Re | Ac | Re | Ac | Re | ||
| 1 FS-1Table Note 28 | Fecal material | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| FS-2 | Ingesta | 1 | 2 | 1 | 2 | 1 | 2 | 2 | 3 | 2 | 3 | 1 | 2 |
| FS-3 | Gastro-intestinal Tract | 1 | 2 | 1 | 2 | 2 | 3 | 2 | 3 | 2 | 3 | 3 | 4 |
| DC-1Table Note 29 | Localized pathology | 0 | 1 | 1 | 2 | 0 | 1 | 0 | 1 | 1 | 2 | 0 | 1 |
Table Notes
- Table Note 28
-
cph = carcasses per hour
- Table Note 29
-
FS = Food safety;
Carcass dressing standards (CDS) decision tree
Each element of the process control and their interaction are explained below and are presented in the decision tree.
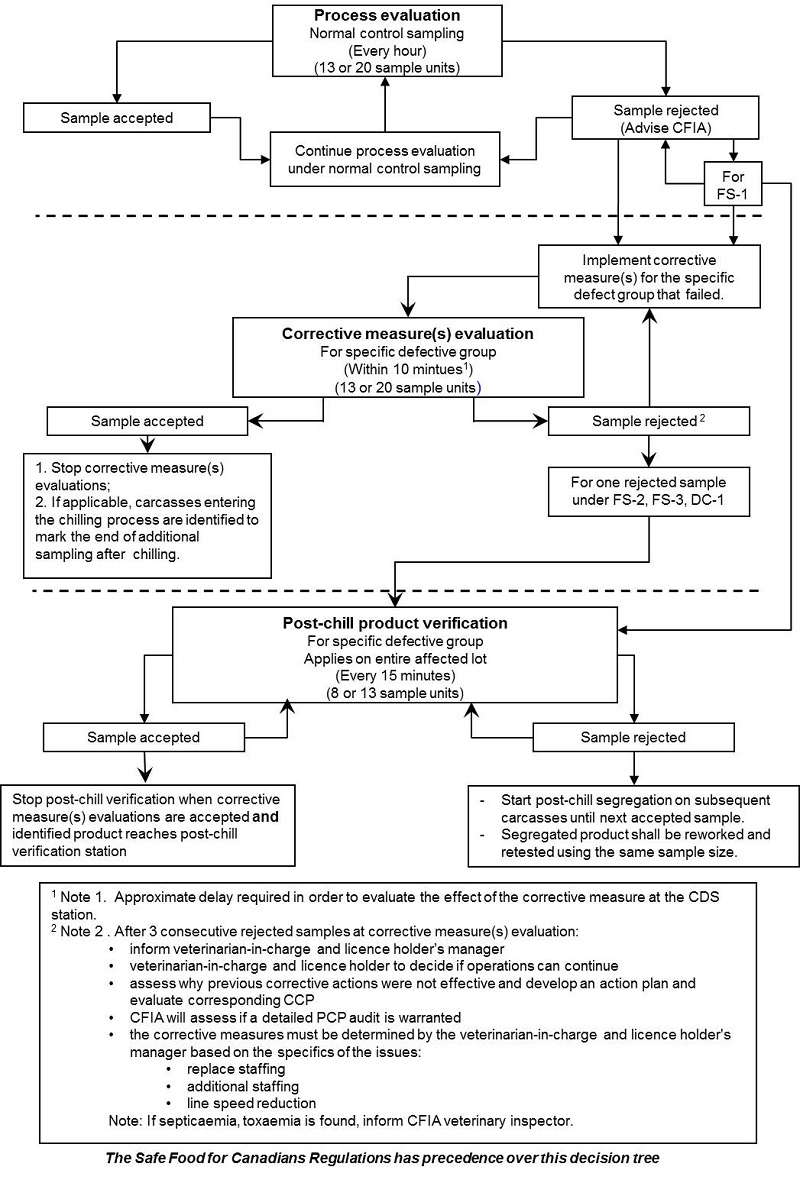
Description for Carcass dressing standards (CDS) decision tree
Step 1: Process evaluation
With Process evaluation under normal control sampling, done every hour for 13 or 20 sample units, there are two possibilities:
- Sample accepted, which leads to continue Process evaluation under normal control sampling, which returns you to the beginning.
Sample rejected: Advise the Canadian Food Inspection Agency, implement corrective measure(s) for the specific defect group that failed, and proceed to Step 2: Corrective measure(s) evaluation, which leads to:
- Continue Process Evaluation under Normal Control Sampling, which returns you to the beginning
- Implement corrective measures for specific defect group, which leads to Step 2: Corrective Measure(s) Evaluation
For Food safety-1, go directly to Step 3: Post-chill product verification
Step 2: Corrective measure(s) evaluation
With Corrective measure evaluation for a specific defective defect group, within 10 minutes (see Note 1) for 13 or 20 sample units, there are two possibilities:
Sample accepted:
- Stop corrective measure(s) evaluations;
- If applicable, carcasses entering the chilling process are identified to mark the end of additional sampling after chilling.
or
Sample rejected (see Note 2):
For one rejected sample under Food Safety-2, Food Safety-3, Dressing Condition-1, proceed to Step 3: Post-chill Product Verification
Step 3: Post-chill product verification
Post-chill product verification, which is for a specific defective defect group, applies on the entire affected lot, is done every 15 minutes and requires 8 or 13 sample units, there are two possibilities:
Sample accepted: Stop post-chill verification when corrective measure(s) evaluations are accepted and identified product reaches post-chill verification station ;
or
- Sample rejected:
- Start post-chill segregation on subsequent carcasses until next accepted sample.
- Segregated product shall be reworked and retested using the same sample size.
Note 1
Approximate delay required in order to evaluate the effect of the corrective measure at the CDS station.
Note 2
After 3 consecutive rejected samples at corrective measure(s) evaluation:
- inform veterinarian-in-charge and licence holder's manager
- veterinarian-in-charge and licence holder to decide if operations can continue
- assess why previous corrective actions were not effective and develop an action plan and evaluate corresponding CCP
- CFIA will assess if a detailed PCP audit is warranted
- the corrective measures must be determined by the veterinarian-in-charge and licence holder's manager based on the specifics of the issues:
- replace staffing
- additional staffing
- line speed reduction
Note
If septicaemia, toxaemia is found, inform CFIA veterinary inspector.
The Safe Food for Canadians Regulations has precedence over this decision tree.
Recording CDS defects
A separate CDS Defects Log must be used for each species of poultry.
For abattoirs with more than one shift per day, test results for each shift must be considered independently because of differences of personnel and supervisors between shifts and test results for each shift must be considered independently. Thus, test results must be recorded on separate CDS Defects Log.
Carcasses are only scored as "defective carcass" for the presence of any distinguishable defect listed in the previous table "CDS Defects". Therefore a carcass showing evidence of the same defect at multiple locations should be recorded as one defective carcass for that specific defect.
Furthermore, a carcass showing multiple defects under the same defect group is only scored as one defective carcass, therefore one carcass having a lung and a bruise = one defective carcass. Also, a carcass with defects under different defect groups is scored as one defective carcass for each of the corresponding groups, therefore a carcass with salpingitis and a 20 mm bruise = one (1) DC-1 defect and one (1) DC-2 defect.
Defective carcasses are grouped by defect into different specified categories. The sample must give the total number of defective carcasses according to each group of defects.
Defects are scored, a total score is determined and acceptability is determined by comparing the score to the applicable acceptance and rejection numbers.
Process out of control – action to be taken
The Carcass Dressing Standards are designed to provide feedback on a lot acceptance basis. They give the licence holder the information needed to respond to and to correct the process. When required, implementing effective corrective measures should prevent the need for post-chill product verification and off-line rework.
Process evaluation
During the process evaluation, each defect group is to be monitored independently of the other defect groups, using the same sample.
When a zero tolerance defect (FS-1, Faecal material) is detected, immediate corrective measure(s) and immediate post-chill product verification must be initiated as per the following sections.
Note
Scheduled randomized hourly pre-chill process evaluation tests must continue independently from corrective measure(s) evaluation tests and post chill product verification tests.
CFIA staff must be informed by the licence holder after each rejected sample.
Corrective measure(s) evaluation
When the test result indicates that the process is under questionable control for a specific defect group, corrective measures must be implemented for this specific defect group and additional on-line testing is required.
Once corrective measures have been implemented, the efficacy of these measures must be evaluated and the evaluation must only apply to the defect group(s) under questionable control. Corrective measures and additional corrective measure(s) evaluations are required until one (1) accepted sample demonstrates conformance to the standard. Line speed reductions would only be applicable as part of corrective measures.
If corrective measures have been accepted while post-chill product verification is been performed, carcasses entering the chilling process are identified to mark the end of additional sampling at the post-chill verification station.
Note
Maximum of five (5) failed tests during MPIP phase-in period and three (3) failed tests after MPIP phase-in period after which the corrective measures by the licence holder must be determined by the veterinary inspector on a case-by-case basis:
- Replace staffing
- Additional staffing
- Line speed reduction
Post-chill product verification
Immediate post-chill product verification and potential product rework is required when one (1) corrective measure(s) evaluation has been rejected in the case of food safety defects and localized pathology defects (FS-1, FS-2, FS-3 and DC-1). The verification is required only for the specific defect group(s) which causes the initial sample to be rejected.
The post-chill verification must cover the entire amount of production or lot that was determined to be out of compliance at the pre-chill station (corrective measure(s) evaluation). If a post-chill product verification sample is rejected, the Establishment's monitor will then identify the affected product so that it may be segregated and accumulated for rework (see reference on the following "CDS Defect Decision Tree").
Other considerations
The licence holder must have a written method that will clearly indicate how plant personnel will identify lots of carcasses requiring rework, isolate these lots, rework the carcasses, and verify that the rework is satisfactory.
Once a specified lot has been reworked for the appropriate defects, a rework verification test must be performed using the appropriate pass and fail criteria for that specific defect group. An accepted sample will result in releasing the retained lot.
The Safe Food for Canadians Regulations (SFCR) has precedence over the CDS decision tree (corrective measures can be mandated at any time by the veterinary inspector).
Verification by the CFIA
When requested, the licence holder must assist the CFIA staff to ensure that the Carcass Dressing Standards (CDS) process control have been implemented and are being performed according to the licence holder's written program.
CFIA will accomplish this by performing correlation tests with the licence holder's monitor as below:
- Process Evaluation – When the licence holder is monitoring the CDS, CFIA will perform a correlation test at a minimum of once per half shift/evisceration line.
- Corrective Measure(s) evaluation – When the licence holder has implemented the corrective measures, the licence holder must conduct corrective measure evaluation test together as correlation test with CFIA inspector.
CFIA staff may perform an additional correlation at any time as a further assurance of process control or if they feel that standards are not being met for any reason.
| Test type | Process evaluation | Corrective measures evaluation |
|---|---|---|
| Monitoring by the industry monitor | Once per hour | Within 10 minutes after implementing corrective action(s) |
| Verification by CFIA staff | Once per half shift | At all times when corrective measures implemented are evaluated by licence holder. |
The Carcass Dressing Standards (CDS) Decision Tree is to be used by the defect detection standards monitor and presented for reference by the CFIA.
9. MPIP implementation and authorization
The Modernized Poultry Inspection Program is a post mortem examination program as defined in Subdivision J of the Safe Food for Canadians Regulations (SFCR) 160 (1) and Post-mortem Examination Program.
Definitions
- MPIP implementation
- means the process of putting a MPIP decision, project or plan into effect as per this guidance document
- MPIP authorization
- means licence holder receiving CFIA permission to perform post-mortem examination duties (defect detection, rejection and process control monitoring) independently with/without CFIA online presence
- MPIP step
- is a stage in the MPIP implementation process. MPIP has 6 steps.
- MPIP phase
- is a stage in the MPIP authorization. MPIP has 3 phases.
- PRP phase
- is a stage in the PRP authorization. PRP has 3 phases.
Information for CFIA
- A CFIA veterinarian must be present throughout evisceration operations in the establishment.
- A CFIA evisceration floor Inspector must be present on the evisceration floor throughout the evisceration operations in the establishment.
- As per the "Modernized Poultry Inspection Program (MPIP) Certification Program", all employees of the CFIA assigned on a regular basis or providing relief in poultry slaughtering establishments operating under MPIP must be certified.
Information for licence holder
- The licence holder must provide full assistance to CFIA for conducting MPIP assessments (including on-site), MPIP tests and any other duties.
- When operating a licensed poultry slaughter establishment, the establishment may be subject to other regulatory requirements outside the scope of this guidance document (such as PCP). The slaughter establishment and operations must always comply with all regulatory requirements and standards.
MPIP implementation steps
MPIP implementation has been designed in various steps. These steps help CFIA assess licence holder's commitment to the MPIP and its ability to perform examination and rejection duties similar to CFIA inspection staff conducted under similar circumstances.
| MPIP Step | Name of the Step | Estimated time (maximum duration)Table Note 30 |
|---|---|---|
| Before MPIP implementation | ||
| Step 1 | Preliminary period and assessment | Variable |
| Before MPIP authorization | ||
| Step 2 | Phase 1: Preparatory period and assessment | Approximately 1 month (maximum 12 months)Table Note 30 |
| Step 3 | Phase 2: Trial period and assessment | Approximately 4 months (maximum 6 months)Table Note 30 |
| Step 4 | Phase 3: Pre-authorization period and assessmentTable Note 31 | Approximately 3 months (maximum 6 months)Table Note 30 |
| After MPIP authorization | ||
| Step 5 | Post-authorization period and assessmentTable Note 31 | 3 months after Step 4 completion |
| After MPIP implementation | ||
| Step 6 | Annual re-assessmentTable Note 31 | 12 months after Step 4 completion and ongoing |
Table Notes
- Table Note 30
-
A continued commitment to the MPIP is required throughout each step.
- Table Note 31
-
See Poultry Rejection Process – PRP (Section 9.6). The licence holder will start PRP from Step 4 onwards.
-
When starting as new, the poultry slaughter operations must initially comply with a post mortem inspection system which will be performed by CFIA Inspectors, as described under the Poultry Traditional Inspection.
This allows licence holder to learn from the CFIA Inspectors before undertaking equivalent responsibility. The licence holder will be required to take traditional line speed caps into consideration when calculating expected daily kill goals.
Note
As part of outcome based regulations, CFIA can place a licence holder in a specific step. For this a request must be made by licence holder to CFIA, together with required documentation indicating how the previous step(s) have already been completed.
Example
Licence holder may choose to train and accredit their employees using "Training protocol for post-mortem examination program" in another MPIP establishment before employees starting MPIP duties in another/new MPIP establishment. The licence holder may apply to skip Step 2&3 and be ready to be assessed by local CFIA in new MPIP establishments before starting Step 4.
Depending upon request and existing abilities of the licence holder, the CFIA will assess and make a decision to place licence holder in a specific MPIP Step. CFIA will provide written justification.
-
At the completion of each MPIP step CFIA will make a regulatory assessment to
- ensure licence holder meets the requirements of the step
- when the licence holder will be allowed to move to the next step
After the licence holder has fully implemented MPIP and been authorized for conducting MPIP activities, assessment will continue to be performed annually.
The assessments are performed using dedicated checklists designed by the CFIA. Copies of the MPIP checklists (such as assessment, implementation or others) may be obtained from the CFIA veterinary inspector or National Poultry Industry Association.
The licence holder may formally withdraw the MPIP application by sending a written request to the CFIA. At that time, the licence holder will resume traditional Inspection.
9.1 Step 1: Preliminary period and assessment
As MPIP is an extremely involved process for both CFIA and the licence holder, the licence holder must be familiar with all steps of the MPIP and must make a firm commitment for implementing MPIP program.
An existing licence holder (such as establishment operating under traditional inspection) or a new licence holder who is interested in implementing MPIP must submit a letter, on company letterhead, of request to the CFIA's Director of Operations with a copy to the VIC (for existing establishments) as per article 160 (1) of the SFCR, "Post mortem Examination Program".
The request must include blueprints and a project description showing the positions/locations of the Inspectors and company employees at the different phases of the implementation and must be presented to the VIC before the beginning of Step 2. The number and location of Inspectors and plant employees may vary from one phase to the next.
Upon receipt of an acceptable request, a 2 station traditional inspection environment, and corresponding line speed, may be allowed at the discretion of the Director of Operations and VIC.
The Director of Operations must review the operator's request and then assemble a Regional Poultry Team (RPT) with the following suggested composition:
- Regional Veterinary Officer
- Veterinarian-in-charge
- Inspection Manager
The RPT will assesses the suitability of the blue print, process flow diagram and the project description submission prior to acceptance based on compliance together with the following criteria:
- facility requirements and line configuration
- training program and work plan for defect detectors, process monitors, rejecters as per "Training protocol for post-mortem examination program"
- a PCP written program
- Poultry Pathogen Reduction Program
Note
For an existing establishment who would like to implement MPIP, the assessment will also include the compliance history of the operator.
CFIA training
As MPIP may also be new to the CFIA employees, CFIA will also need to provide training to CFIA employees as below:
- the veterinary inspector and Inspectors may receive several days of practical training in one or more MPIP establishments
- certified MPIP trainers must organize training workshop(s) to certify CFIA staff and establishment trainers
- as training may affect the start of the Step 2, the licence holder must take this into MPIP consideration
Step 1 completion assessment
The licence holder should inform the veterinary inspector when all the requirements of Step 1 are complete.
Prior to start of Step 2, an assessment will be completed by the CFIA assessment team for this purpose. A copy of this assessment is forwarded to the Director of Operations and the licence holder.
CFIA will use a checklist entitled "MPIP Preliminary Assessment Checklist". The licence holder will receive results from the CFIA assessment. Minor deficiencies may be addressed through acceptable action plans.
Based on the result of this checklist, the RPT will recommend the commencement of Step 2 or that Step 1 be continued pending the completion of all requirements.
The licence holder must review the CFIA assessment, the terms and conditions required to be completed prior to initiating operations in an MPIP environment. CFIA will forward a copy of this assessment to local CFIA management.
Step 1 complete:
- If the request is accepted, the CFIA personnel from the establishment and the licence holder are informed that the request to implement MPIP has been approved by the RPT.
- The licence holder may then schedule a Step 2 (Phase 1) start date.
Step 1 incomplete:
- If the request is refused, the RPT will explain the decision and rationale to the licence holder and local CFIA management.
- There may also be a temporary delay at the start of the Step 2. The licence holder must correct deficiencies before submitting a new request.
- When submitting new request or resubmitting an existing request, the licence holder must correct deficiencies before requesting a CFIA assessment.
9.2 Step 2: Phase 1 – Preparatory period and assessment
Goals of Step 2 (Phase 1)
During Step 2 the licence holder is expected to:
- complete the training and accreditation of establishment trainers and supervisors
- have a sufficient number of defect detectors who have completed training and accreditation
- have a sufficient number of process monitors who have completed training and accreditation and can administer the process control tests ES, PS DDS, and CDS
- implement a Pathogen Reduction Program which is operational and included in the PCP system
- complete modification of the evisceration line and inspection/examination stations so that the CFIA employees and licence holder's employees can complete their duties in Step 3 (Phase 2)
Certified MPIP trainers must organize training workshop(s) to certify CFIA staff.
- The VIC and one or more Inspectors must receive several days of practical training in one or more MPIP plants.
Line speeds during Step 2
- The licence holder must consult with the veterinary inspector about CFIA staffing and maximum line speeds during Step 2. The line speed will depend on the ergonomic facilities and the presentation of the carcasses and corresponding viscera.
- An onsite review by CFIA of the establishment will be required to ensure MPIP facility requirements have been met. Line speeds may be increased during this Step, provided the licence holder has implemented carcass/cavity detection, ES, ES, DDS and CDS by accredited employees.
- The maximum line speed (carcasses per hour – cph) for evisceration procedures must be approved by the veterinary inspector as per the following:
- 7200 cph for chicken
- 6360 cph for light fowl under 2 kg (live weight)
- 5760 cph for heavy fowl over 2 kg (live weight)
- 3600 cph for light turkey at or under 8 kg
- 3300 cph for heavy turkey over 8 kg
CFIA station location and tests
CFIA veterinary inspector will require CFIA stations be located upstream and require trainers, defect detectors be located after CFIA Inspectors until the veterinary inspector is satisfied that trainers, defect detectors are able to perform their duties equivalent to the CFIA Inspectors.
Once the defect detectors start performing to satisfaction of the CFIA veterinary inspector, the carcass and/or cavity defect detectors will exchange the position with the CFIA Inspector(s) responsible for carcass and/or cavity inspection.
Once, the CFIA Inspectors are located downstream to defect detectors, the performance for the carcass and/or cavity defect detectors will be supervised using the DDS test. CFIA Inspectors will continue to provide feedback if there is failure of the DSS test as below:
| Phase | DDS testing mode |
|---|---|
| During Step 2: Phase 1 | CFIA will conduct the DDS tests:
|
Note
DDS testing during this training step will be used only to provide feedback to carcass and/or cavity defect detectors and will not be considered non-compliance to regulatory requirements for carcass, cavity and viscera defect group.
Licence holder employees training
- The licence holder can receive the MPIP industry training material from the CFIA veterinary inspector to train and accredit establishment trainers.
- Training for Evisceration standards (ES), Presentation standards (PS), Defect detection standards (DDS) and the Carcass dressing standards (CDS) must be implemented prior to commencing Step 3
- Once the MPIP establishment trainers have been accredited, establishment trainers are to then train and accredit all other establishment employees as per the "Training protocol for post-mortem examination program".
Exports
If the licence holder desires to remain eligible to export to the United States, a written request must be submitted to the VIC for the CFIA to continue staffing an on-line station called an "export" station (refer to Exports, USA).
Switch to pre-MPIP
The licence holder may switch to a pre-MPIP environment (defect detection, poultry rejection process and process controls) when
- the CFIA operational requirement are met
- correction of any non-conformities identified during Step 1 assessment including requisite amendments to the licence holder's PCP system
- licence holder's employees' level of training and expertise with can facilitate a functional Pre-MPIP environment as determined by the veterinary inspector
- the facility requirements have completed the requirements for MPIP stations in Step 3 (Phase 2)
Step 2 completion assessment
The licence holder should inform veterinary inspector when all the requirements of Step 2 (Phase 1) are complete.
Prior to start of Step 3 (Phase 2), an assessment will be completed by the RPT for this purpose.
CFIA will use a checklist entitled "MPIP Preliminary Assessment Checklist.
The RPT using the checklist entitled "MPIP IMPLEMENTATION ASSESSMENT CHECKLIST Preparatory Period (Phase 1) to Trial Period (Phase 2) Assessment" to evaluate the requirements in order to commence Step 3. The licence holder will receive results from the CFIA assessment.
Based on the result of this checklist, the RPT will recommend the commencement of Phase 2 or that Phase 1 be continued pending the completion of all requirements.
The licence holder must review the CFIA assessment, the terms and conditions required to be completed prior to initiating Step 3. CFIA will forward a copy of this assessment to local CFIA management.
Step 2 complete:
- When Step 2 is completed, the CFIA personnel from the establishment and the licence holder are informed that the request to implement MPIP has been approved by RPT.
- Minor deficiencies may be addressed through action plans acceptable to local CFIA.
- The licence holder may then schedule a Step 3 (Phase 2) start date.
Step 2 incomplete:
- If the request is incomplete, the RPT will explain the decision and rationale to the licence holder and local CFIA management.
- The licence holder must correct deficiencies before submitting a new request. This may temporarily delay the start of the Step 3.
- When submitting a new request or resubmitting an existing request, the licence holder must correct deficiencies before requesting CFIA re-assessment.
9.3 Step 3: Phase 2 – Trial period and assessment
Goals of Step 3 (Phase 2)
During Step 3 licence holder must:
- have establishment trainers request feedback from the CFIA inspection staff on the performance of their defect detectors
- have weekly meetings with the veterinary inspector throughout Step 3 (Phase 2) to discuss any issues related to the ongoing MPIP implementation
- continue to provide same number of on-line CFIA inspection stations as staffed under the licence holder's previous post mortem inspection system
- have all defect detectors pass four (4) additional on-line tests as per the "Training protocol for post-mortem examination program"
- if switching from Traditional inspection to MPIP, and the licence holder desires to remain eligible to export to the United States, the licence holder must submit a written request, on company letterhead, to the VIC, to request continued staffing of an "export" station (refer to Chapter 11, Exports, USA)
CFIA station and tests
CFIA veterinary inspector will require CFIA stations be located upstream and require trainers, defect detectors be located after CFIA Inspectors until the veterinary inspector is satisfied that trainers, defect detectors are able to perform their duties equivalent to the CFIA Inspectors.
Once the defect detectors start performing to satisfaction of the CFIA veterinary inspector, the viscera defect detectors will exchange the position with the CFIA Inspector(s) responsible for viscera inspection.
Once, the CFIA Inspectors are located downstream to defect detectors, the performance for the viscera defect detection will be supervised using the DDS test. CFIA Inspectors will continue to provide feedback if there is failure of the DSS test as below:
| Phase | DDS testing mode |
|---|---|
| During Step 3: Phase 2 |
CFIA will conduct the DDS tests:
|
Note
DDS testing during this training step will be used only to provide feedback to viscera defect detectors and will not be considered non-compliance to regulatory requirements for viscera defect group.
Step 3 completion assessment
The licence holder should inform the veterinary inspector when all the requirements of Step 3: Phase 2 is complete.
Prior to start of Step 4: Phase 3, an assessment will be completed by the RPT for this purpose.
CFIA will use a checklist entitled "MPIP Preliminary Assessment Checklist.
The RPT will use the checklist entitled, "MPIP Compliance and Verification Checklist Trial Period (2nd Phase) to Implementation Period (3rd Phase) & Annual Verification" to evaluate the requirements in order to commence Step 4. The licence holder will receive results from the CFIA assessment.
Based on the result of this checklist, the RPT will recommend the commencement of Step 4 (Phase 3) or that Step 3 (Phase 2) be continued pending the completion of all requirements.
The licence holder must review the CFIA assessment, the terms and conditions required to be completed prior to initiating Step 4. CFIA will forward a copy of this assessment to local CFIA management.
Step 3 complete:
- When Step 3 is completed, the CFIA personnel from the establishment and the licence holder are informed that the request to implement MPIP has been approved by RPT.
- Minor deficiencies may be addressed through action plans acceptable to local CFIA.
- If performance is acceptable, the licence holder may enter Step 4 (Phase 3)
- The licence holder may then schedule a Step 3 (Phase 3) start date.
Step 3 incomplete:
- If the request is incomplete, the RPT will explain the decision and rationale to the licence holder and local CFIA management.
- The request may also temporarily delay the start of the Step 4. The licence holder must correct the deficiencies before submitting a new request.
- When submitting a new request or resubmitting an existing request, the licence holder must correct deficiencies before requesting CFIA re-assessment.
- If the licence holder does not exhibit optimum performance, then the Step 3 must continue until the performance is acceptable or the licence holder formally withdraws its MPIP application.
9.4 Step 4: Phase 3 – Pre-authorization implementation period an assessment
Goals of step 4 (Phase 3)
- CFIA will remove the line speed limits.
- line speeds are determined by the performance of the industry defect detectors and by compliance with MPIP requirements.
- the licence holder may implement poultry On-line reprocessing and reconditioning or Modified on-line reprocessing and reconditioning in Phase-3. See guidance under "Poultry Off-line and On-line Reprocessing and Reconditioning Procedures".
- Prepare implementation of the Poultry rejection process (PRP).
CFIA stations and tests
During this stage licence holder may notice that CFIA staff may undergo operational modifications according to the roles and responsibilities of CFIA staff under Step 4 (Phase 3):
- the CFIA staffing levels may be reduced
- cost recovery fees must be recalculated based on remaining CFIA inspection stations and inspection staff tasks
The CFIA Inspectors (under veterinary inspector supervision) will start supervising defect detectors using the DDS test as below:
| Phase | DDS testing mode |
|---|---|
| During and after Step 4: Phase 3 | Implement the DDS test for carcass, cavity and viscera defect groups. |
Step 4 completion assessment
Note
Step 4 (Phase 3) must last a minimum of 3 months probationary period.
The licence holder should inform veterinary inspector when all the requirements of Step 4 (Phase 3) are complete.
Prior to start of Step 5, an assessment will be completed by the RPT for this purpose.
CFIA will use a checklist entitled, "MPIP Compliance and Verification Checklist Trial Period (2nd Phase) to Implementation Period (3rd Phase) & Annual Verification" to evaluate the requirements in order to commence Step 5. The licence holder will receive results from the CFIA assessment.
Based on the result of this checklist, the RPT will recommend the commencement of Step 5 or that Step 4 (Phase 3) be continued pending the completion of all requirements.
The licence holder must review the CFIA assessment, the terms and conditions required to be completed prior to initiating Step 5. CFIA will forward a copy of this assessment to local CFIA management.
Step 4 complete:
- When Step 4 is completed, the CFIA personnel from the establishment and the licence holder are informed that the request to implement MPIP has been approved by the RPT.
- Minor deficiencies may be addressed through action plans acceptable to local CFIA.
- If performance is acceptable, the licence holder may enter Step 5
- The licence holder may then schedule a Step 5 start date.
Note
At conclusion of Step 4 (Phase 3), the licence holder receives full CFIA authorization towards MPIP.
Step 4 incomplete:
- If the request is incomplete, the RPT will explain the decision and rationale to the licence holder and local CFIA management.
- The request may also temporarily delay the start of the Step 4, provided licence holder must correct deficiencies before submitting a new request.
- When submitting a new request or resubmitting an existing request, the licence holder must correct deficiencies before requesting CFIA re-assessment.
- If the licence holder does not exhibit optimum performance, then Step 4 must continue until the performance is acceptable or the licence holder formally withdraws its MPIP application.
- If the licence holder does not continue to progress towards the completion of MPIP authorization the CFIA has the authority to discontinue the process and the licence holder will need to revert to Traditional Inspection.
9.5 Step 5: Post-authorization implementation period and assessment
Goals of Step 5
This ensures continuous commitment of licence holder.
Step 5 completion assessment
Note
Step 5 must last a minimum of 3 months after completion of the Step 4.
Complete all requirements as written in Section 9.4 "Step 4 completion assessment"
9.6 Step 6: Annual re-assessment
Goals of Step 6
This ensures continuous commitment of licence holder.
Step 6 completion assessment
Note
Step 6 is performed after 12 months after completion of the Step 5.
This step is self-assessment by the licence holder using entitled "MPIP Compliance and Verification Checklist Trial Period (2nd Phase) to Implementation Period (3rd Phase) & Annual Verification".
A copy of completed self-assessment will be provided to the VIC for examination.
Note
Although, self-serve annual re-assessment will be done by the licence holder, CFIA reserves right to conduct re-assessment at any time.
CFIA staff will use CVS Task 1.5.05 to verify the compliance with the performance of the MPIP tests and associated record keeping. In addition, a review is done of the training and accreditation of the staff responsible for these activities. This task is done annually.
10. Poultry rejection process (PRP) implementation and authorization
PRP will be implemented in the MPIP establishment when
- The licence holder has qualified for Phase 3: Pre-authorization period and assessment of MPIP;
- CFIA staff and designated industry employees were trained on the most recent poultry disposition policies, DDS and CDS; and
- DDS and CDS are fully implemented.
The PRP must be implemented in three (3) phases in abattoirs that have qualified for Phase 3 of the MPIP implementation.
The following table summarizes the preliminary activities, three (3) phases for implementation and post-implementation activities for the PRP:
| Phase | Activities | Estimated duration |
|---|---|---|
| Before PRP implementation | ||
| PRP Preliminary Activities | Ensure that CFIA staff and designated industry employees are trained on the most recent poultry disposition policies | Variable for each licence holder |
| Before PRP authorization and implementation | ||
| PRP Phase 1 Preparatory Period |
Train CFIA veterinary inspector for collecting and recording data required for Phase 2. Complete training and accreditation of industry trainers and rejecters for performing rejections. | 1 – 2 weeks |
| PRP Phase 2 Trial Period |
CFIA veterinary inspector mentor/coach the rejecters and collect data for a plant specific reference line ("before" data) for false positives and negatives. | 4 – 6 weeks, minimum of 20 consecutive production shifts |
| PRP Phase 3 Pre-authorization Implementation Period |
Industry performs rejections, collects and collates the rejection data and issues disposition reports. On-site CFIA inspection staff verifies the rejection process. | Minimum of 3 months |
| After PRP authorization and implementation | ||
| PRP Post-authorization implementation | Industry performs rejections, collects and collates the rejection data and issues disposition reports. On-site CFIA inspection staff verifies the rejection process. | Ongoing |
10.1 PRP preliminary activities
Establishment must be in MPIP Phase 3 to officially start the PRP.
The licence holder will make a request to the VIC for training of the licence holder's trainers on the latest poultry disposition policy for all poultry species processed in the establishment.
If there are policy changes implemented by the CFIA, the licence holder's trainers must update/retrain the remaining defect detectors by using the latest versions of the CFIA developed industry training modules for carcass, cavity and viscera defect detection.
Note
The PRP relies on the efficient defect detection process carried out by the licence holder's defect detectors as per CFIA disposition policy. This reduces the number of carcasses and viscera at the rejection station with false positive and false negative test results.
10.2 PRP Phase 1 – Preparatory period
During PRP Phase 1:
- Licence holder is responsible for:
- providing trainer's names and making them available to VIC
- maintaining up to date training records
- presenting a written description of the facilities and Standard Operating Procedures (SOPs) for the rejection process to the VIC
- preparing to issue rejection reports
- preparing for benchmarking total rejections for farm pathology
- Licence holder's trainers are responsible for:
- training and accreditation of rejecters and supervisors
- Licence holder's rejecter(s) is/are responsible for:
- being trained and accredited
Licence holder must ensure that at least two (2) trainers and monitors per shift get trained and accredited according to the training guidance contained in "Training protocol for post-mortem examination program".
After the licence holder's trainers are trained, they will then train and accredit the remaining industry defect detectors and other personnel performing or assessing rejections (such as supervisor and carcass, cavity and viscera defect detectors who will be performing rejections).
The licence holder must prepare to issue a condemnation/rejection report during PRP Phase 1. In particular, any computer software and a database needed to issue such reports should be created or enhanced such that all the required information can be entered. The licence holder must be able to track the total rejections for farm origin pathology, so that the information can be entered onto each condemnation/rejection report.
The farm origin pathology has been defined as DDS defects in the Defect Detection Standards (DDS) program section 8.3.2. If any other pathology is noticed, the CFIA veterinary inspector should be notified.
Note
At the same time CFIA will also conduct training of CFIA employees. When requested, the licence holder must provide assistance to the CFIA by providing training facilities.
Phase 2 should begin as soon as sufficient industry personnel have been accredited for performing rejections.
10.3 PRP Phase 2 – Trial period
During PRP Phase 2:
- Licence holder is responsible for:
- assessing if the rejection process and/or facility improvements are required
- addressing any outstanding training, facilities, technical or other issues for an efficient rejection process
- having rejecter available for the Veterinary Inspector performing the Rejection Correlation Tests (RCTs)
- presenting each carcass to the CFIA Evisceration Floor Inspector which was removed by the defect detectors for suspected pathology and was not rejected by the rejecter
- Trainers are responsible for:
- continuing training and accreditation as needed
- addressing any outstanding training issues
- Rejecter(s) is/are responsible for
- practising rejections
Throughout PRP Phase 2, the CFIA veterinary inspector will mentor and coach the licence holder's rejecters full-time while the rejecters gain practical experience.
Throughout Phase 2, all carcasses with suspected generalized pathology, which are removed from the line by the defect detectors, must be presented to the CFIA veterinary inspector by the rejecter in a manner acceptable to the VIC (either manually or on CFIA accepted facilities) on a truck/lot basis for Veterinary diagnosis. CFIA veterinary inspector will administer a Rejection Correlation Test (RCT) on each carcass and viscera at the Veterinary disposition Station. This test is used to generate data which is used by the VIC to construct a variety of charts and graphs for use during PRP Phase 3 and PRP Post-Implementation.
During the RCT, both the licence holder and the CFIA veterinary inspector will track the false positives, false negatives and questionable carcasses (as explained under the RCT) for each truck/lot. When it appears that the performance of the newly trained rejecters has passed through the learning curve and has plateaued/stabilized, then data on false positives, false negatives and questionable carcasses must be collected for another 20 consecutive production shifts (each shift is considered separately) after which the CFIA will perform a formal audit to assess if the licence holder qualifies to advance to PRP Phase 3 of implementing the PRP.
The rejecter must dispose of the carcass as instructed by the veterinary inspector and, if rejected, must learn to record the carcass in the correct category (see section 7.2).
CFIA veterinary inspector will track information on the type and number of questionable carcasses separately to enhance training of the rejecters during phase 2. CFIA will require licence the holder to share the rejection data monthly with CFIA.
The CFIA will also continue to collate the disposition data for submission to the CFIA HQ at the end of each month. This data is collected by CFIA on behalf of Agriculture and Agri-Food Canada and is used to generate CFIA's monthly "Ante Mortem and Post Mortem Inspection Report".
The CFIA review team may comprise of specialists and formal establishment auditors which may last more than a day. CFIA completes the "Poultry Rejection Process (PRP) Compliance Checklist, Phase 2 (Trial Period) to Phase 3 (Pre-authorization Implementation Period)".
PRP Phase 3 may commence when all deficiencies identified during the audit/review which might affect the performance of the rejecters have been corrected.
10.4 PRP Phase 3 – Pre-authorization implementation period
During PRP Phase 3:
- Licence holder is responsible for
- Rejection Process
- Initiating corrective actions as required
- Trainers are responsible for
- Continuing training and accreditation as needed
- Maintaining up to date training records
- Addressing any outstanding training issues
- Rejecter(s) is/are responsible for
- Performing PRP tasks (for example, sorting suspect carcasses, etc.)
- Collecting and collating data
- Setting aside all evaluated suspect carcasses from each lot selected for an RCT
- Presenting all questionable carcasses to a CFIA veterinary inspector
- Setting aside eviscerated and passed carcasses for CFIA inspection
- Setting aside rejected carcasses for CFIA inspection if instructed by a CFIA veterinary inspector
- Notify a CFIA veterinary inspector if presented with a lot with unusual pathology
In PRP Phase 3, the licence holder will commence the following tasks:
- performing rejections (without a detailed Veterinary inspection for each carcass removed from the evisceration line for suspected farm origin pathology)
- assist CFIA veterinary inspector in performing an RCT at any time and/or when instructed by CFIA veterinarian
- set aside all rejected carcasses and corresponding viscera
- questionable carcasses and viscera must be set aside for a detailed Veterinary inspection and feedback to rejecters and CFIA Inspectors
- record rejection data
Note
Each carcass and corresponding viscera removed by the defect detectors for suspected pathology, that is not rejected by the rejecter, must be set aside in a designated location and must be presented for CFIA Inspector before returning to the food chain.
PRP Pre-authorization Implementation Period is considered to be completed after 3 consecutive months of consistently satisfactory performance as assessed by the VIC. This assessment will include the results of the RCT performed by a CFIA veterinarian for the rejection process during PRP Phase 2 and PRP Phase 3.
10.5 Post-authorization implementation
During the Post-authorization implementation:
- Licence holder is responsible for
- All activities as per Phase 3
- Reviewing rejection data (PRP Excel Spreadsheet) and taking appropriate action(s) as required
- Trainers are responsible for
- Performing all activities as per Phase 3
- Updating rejecters on the rejection policy modifications as required
- Rejecter(s) is/are responsible for
- Performing all activities as per Phase 3
The PRP is considered to be fully implemented after Phase 3 is successfully completed.
The CFIA inspection staff will continue to perform the same activities described in Phase 3 of implementing the PRP including at minimum randomly selecting one lot per half shift for an RCT by a CFIA veterinary inspector. The VIC will provide the establishment operator with an electronic copy of the excel file containing the data collected during the RCTs and the resulting control graphs once per week on an ongoing basis. Any emerging trend(s) evident on one or more graphs will be discussed with the licence holder.
The CFIA will then authorize the licence holder to operate under the PRP as part of the MPIP.
Additional Guidance:
- Guidance on Canadian Food Inspection Agency inspection stations for slaughter operation of food animals and meat products
- Poultry Pathogen Reduction Program
- Poultry Traditional Inspection
- Training protocol for post-mortem examination program
- Date modified: